当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experiment and Simulations for the Thermal Safety of the Nitration Reaction Liquid of the Final State in the Synthesis Process of N-Nitrodihydroxyethyl Dinitrate (DINA)
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.oprd.1c00172 Yanlong Zhu 1 , Jing An 1 , Jing Zhou 1 , Xiaofeng Wang 1 , Hai Chang 1 , Li Ding 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.oprd.1c00172 Yanlong Zhu 1 , Jing An 1 , Jing Zhou 1 , Xiaofeng Wang 1 , Hai Chang 1 , Li Ding 1
Affiliation
|
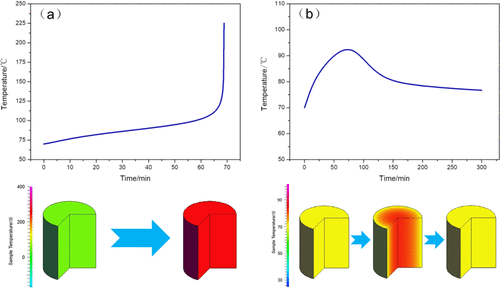
In the synthesis process of N-nitrodihydroxyethyl dinitrate (DINA) with the HNO3–Mg(NO3)2 method, the thermal stability of the nitration reaction liquid of the final state is poor, which leads to the thermal runaway of the entire reaction system easily. The research on the thermal runaway reaction under actual reaction conditions indicates high consumption and high risk. In this article, thermal decomposition behavior and isothermal thermal decomposition kinetics of the nitration reaction liquid of the final state in the synthesis process of DINA were investigated by differential scanning calorimetry (DSC) and microcalorimetry. The mechanism of the stability of the nitration reaction liquid was explored. The thermal safety of the material in a large-scale reactor was simulated and predicted by a thermal simulation software on the basis of kinetic parameters including activation energy, pre-exponential factors, and mechanistic functions. These findings not only avoid the risk and consumption of large-size materials but also guide their application in process optimization, inherent safety design, and handling.
中文翻译:

N-硝基二羟乙基二硝酸酯(DINA)合成过程中终态硝化反应液的热安全性实验与模拟
在N-硝基二羟乙基二硝酸酯(DINA)与HNO 3 -Mg(NO 3 ) 2的合成过程中方法,终态硝化反应液的热稳定性差,容易导致整个反应体系热失控。对实际反应条件下的热失控反应的研究表明,高消耗和高风险。本文采用差示扫描量热法(DSC)和微量热法研究了DINA合成过程中终态硝化反应液的热分解行为和等温热分解动力学。探讨了硝化反应液稳定性的机理。利用热模拟软件根据活化能、指前因子、和机械功能。这些发现不仅避免了大尺寸材料的风险和消耗,而且还指导了它们在工艺优化、固有安全设计和处理方面的应用。
更新日期:2021-09-17
中文翻译:

N-硝基二羟乙基二硝酸酯(DINA)合成过程中终态硝化反应液的热安全性实验与模拟
在N-硝基二羟乙基二硝酸酯(DINA)与HNO 3 -Mg(NO 3 ) 2的合成过程中方法,终态硝化反应液的热稳定性差,容易导致整个反应体系热失控。对实际反应条件下的热失控反应的研究表明,高消耗和高风险。本文采用差示扫描量热法(DSC)和微量热法研究了DINA合成过程中终态硝化反应液的热分解行为和等温热分解动力学。探讨了硝化反应液稳定性的机理。利用热模拟软件根据活化能、指前因子、和机械功能。这些发现不仅避免了大尺寸材料的风险和消耗,而且还指导了它们在工艺优化、固有安全设计和处理方面的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号