当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biodistribution of Adeno-Associated Virus Serotype 5 Viral Vectors Following Intrathecal Injection
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-08-30 , DOI: 10.1021/acs.molpharmaceut.1c00252 Kelsey R Pflepsen 1 , Cristina D Peterson 2 , Kelley F Kitto 2 , Maureen S Riedl 2 , R Scott McIvor 3 , George L Wilcox 2, 4, 5 , Lucy Vulchanova 2 , Carolyn A Fairbanks 1, 2, 4
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-08-30 , DOI: 10.1021/acs.molpharmaceut.1c00252 Kelsey R Pflepsen 1 , Cristina D Peterson 2 , Kelley F Kitto 2 , Maureen S Riedl 2 , R Scott McIvor 3 , George L Wilcox 2, 4, 5 , Lucy Vulchanova 2 , Carolyn A Fairbanks 1, 2, 4
Affiliation
|
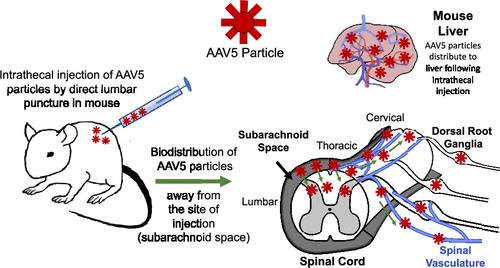
The pharmacokinetic profile of AAV particles following intrathecal delivery has not yet been clearly defined. The present study evaluated the distribution profile of adeno-associated virus serotype 5 (AAV5) viral vectors following lumbar intrathecal injection in mice. After a single bolus intrathecal injection, viral DNA concentrations in mouse whole blood, spinal cord, and peripheral tissues were determined using quantitative polymerase chain reaction (qPCR). The kinetics of AAV5 vector in whole blood and the concentration over time in spinal and peripheral tissues were analyzed. Distribution of the AAV5 vector to all levels of the spinal cord, dorsal root ganglia, and into systemic circulation occurred rapidly within 30 min following injection. Vector concentration in whole blood reached a maximum 6 h postinjection with a half-life of approximately 12 h. Area under the curve data revealed the highest concentration of vector distributed to dorsal root ganglia tissue. Immunohistochemical analysis revealed AAV5 particle colocalization with the pia mater at the spinal cord and macrophages in the dorsal root ganglia (DRG) 30 min after injection. These results demonstrate the widespread distribution of AAV5 particles through cerebrospinal fluid and preferential targeting of DRG tissue with possible clearance mechanisms via DRG macrophages.
中文翻译:

鞘内注射后腺相关病毒血清型 5 病毒载体的生物分布
鞘内给药后 AAV 颗粒的药代动力学特征尚未明确定义。本研究评估了小鼠腰椎鞘内注射后腺相关病毒血清型 5 (AAV5) 病毒载体的分布概况。在单次推注鞘内注射后,使用定量聚合酶链式反应 (qPCR) 测定小鼠全血、脊髓和外周组织中的病毒 DNA 浓度。分析了全血中 AAV5 载体的动力学以及随着时间的推移在脊髓和外周组织中的浓度。AAV5 载体在注射后 30 分钟内迅速分布到脊髓、背根神经节和全身循环的所有水平。全血中的载体浓度在注射后 6 小时达到最大,半衰期约为 12 小时。曲线数据下的面积显示分布到背根神经节组织的载体浓度最高。免疫组织化学分析显示,注射后 30 分钟,AAV5 颗粒与脊髓软脑膜和背根神经节 (DRG) 中的巨噬细胞共定位。这些结果表明 AAV5 颗粒在脑脊液中的广泛分布和通过 DRG 巨噬细胞可能的清除机制优先靶向 DRG 组织。免疫组织化学分析显示,注射后 30 分钟,AAV5 颗粒与脊髓软脑膜和背根神经节 (DRG) 中的巨噬细胞共定位。这些结果表明 AAV5 颗粒在脑脊液中的广泛分布和通过 DRG 巨噬细胞可能的清除机制优先靶向 DRG 组织。免疫组织化学分析显示,注射后 30 分钟,AAV5 颗粒与脊髓软脑膜和背根神经节 (DRG) 中的巨噬细胞共定位。这些结果表明 AAV5 颗粒在脑脊液中的广泛分布和通过 DRG 巨噬细胞可能的清除机制优先靶向 DRG 组织。
更新日期:2021-10-04
中文翻译:

鞘内注射后腺相关病毒血清型 5 病毒载体的生物分布
鞘内给药后 AAV 颗粒的药代动力学特征尚未明确定义。本研究评估了小鼠腰椎鞘内注射后腺相关病毒血清型 5 (AAV5) 病毒载体的分布概况。在单次推注鞘内注射后,使用定量聚合酶链式反应 (qPCR) 测定小鼠全血、脊髓和外周组织中的病毒 DNA 浓度。分析了全血中 AAV5 载体的动力学以及随着时间的推移在脊髓和外周组织中的浓度。AAV5 载体在注射后 30 分钟内迅速分布到脊髓、背根神经节和全身循环的所有水平。全血中的载体浓度在注射后 6 小时达到最大,半衰期约为 12 小时。曲线数据下的面积显示分布到背根神经节组织的载体浓度最高。免疫组织化学分析显示,注射后 30 分钟,AAV5 颗粒与脊髓软脑膜和背根神经节 (DRG) 中的巨噬细胞共定位。这些结果表明 AAV5 颗粒在脑脊液中的广泛分布和通过 DRG 巨噬细胞可能的清除机制优先靶向 DRG 组织。免疫组织化学分析显示,注射后 30 分钟,AAV5 颗粒与脊髓软脑膜和背根神经节 (DRG) 中的巨噬细胞共定位。这些结果表明 AAV5 颗粒在脑脊液中的广泛分布和通过 DRG 巨噬细胞可能的清除机制优先靶向 DRG 组织。免疫组织化学分析显示,注射后 30 分钟,AAV5 颗粒与脊髓软脑膜和背根神经节 (DRG) 中的巨噬细胞共定位。这些结果表明 AAV5 颗粒在脑脊液中的广泛分布和通过 DRG 巨噬细胞可能的清除机制优先靶向 DRG 组织。









































 京公网安备 11010802027423号
京公网安备 11010802027423号