Energy Storage Materials ( IF 18.9 ) Pub Date : 2021-08-31 , DOI: 10.1016/j.ensm.2021.08.040 Dandan Yu 1 , Hua Wang 2 , Wei Zhang 1 , Huafeng Dong 3 , Qiaonan Zhu 2 , Jie Yang 2 , Shaoming Huang 1
|
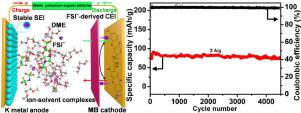
Redox-active organic cathodes are promising high-capacity and low-cost electrode materials for potassium-ion batteries (KIBs), but usually suffer from poor cyclability due to the severe dissolution and notorious K dendrites. Herein, the effects of salt concentrations and solvents on the performance of potassium-organic batteries based on a typical phenothiazine derivative, methylene blue (MB), are explored, and the high-concentration potassium bis(fluorosulfonyl)amide-based ether electrolyte is versatile to achieve long lifespan. The high donor number and strong solvation ability of ether solvent endow MB cathode with high voltage plateaus and reversible capacity as compared to the carbonate counterpart. Moreover, the enhanced interactions of K+-ether and anion-participated ion-ether complexes in concentrated electrolyte not only decrease the amount of free solvent, but also contribute to forming highly ionic-conducting and thin anion-derived interphase layers, which effectively mitigate the shuttle effect and inhibit K dendrite growth. Consequently, MB cathode exhibits a high capacity of 139.5 mAh g−1 after 500 cycles at 0.1 A g−1, and long-term cycling stability for 4500 cycles. The good performance of full cells matched with graphite anode further demonstrates the feasibility of MB as KIB cathode. This work highlights the roles of ion-solvent chemistry in stabilizing small-molecule organic cathodes, which may bring new insights in the electrolyte design for wide organic electrode-based battery systems.
中文翻译:

阐明离子溶剂化学在稳定钾离子电池小分子有机正极中的作用
氧化还原活性有机正极是一种很有前途的高容量、低成本的钾离子电池(KIBs)电极材料,但由于严重的溶解和臭名昭著的 K 枝晶,通常循环性较差。在此,探讨了盐浓度和溶剂对基于典型吩噻嗪衍生物亚甲蓝 (MB) 的钾有机电池性能的影响,以及高浓度双(氟磺酰基)酰胺钾基醚电解质是通用的以达到长寿命。与碳酸盐对应物相比,醚溶剂的高供体数和强溶剂化能力使 MB 正极具有高电压平台和可逆容量。此外,K +的增强相互作用浓电解质中-醚和阴离子参与的离子-醚络合物不仅减少了游离溶剂的量,而且有助于形成高离子导电性和薄的阴离子衍生界面层,有效减轻穿梭效应并抑制K枝晶生长. 因此,MB 正极在 0.1 A g -1 下500 次循环后表现出 139.5 mAh g -1的高容量,以及 4500 次循环的长期循环稳定性。与石墨阳极匹配的全电池的良好性能进一步证明了 MB 作为 KIB 阴极的可行性。这项工作强调了离子溶剂化学在稳定小分子有机正极方面的作用,这可能为基于有机电极的电池系统的电解质设计带来新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号