当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cα-Methyl-l-valine: A Preferential Choice over α-Aminoisobutyric Acid for Designing Right-Handed α-Helical Scaffolds
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.biochem.1c00340 Raja Banerjee , Tridip Sheet , Srijan Banerjee , Barbara Biondi 1, 2 , Fernando Formaggio 1, 2 , Claudio Toniolo 1, 2 , Cristina Peggion 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.biochem.1c00340 Raja Banerjee , Tridip Sheet , Srijan Banerjee , Barbara Biondi 1, 2 , Fernando Formaggio 1, 2 , Claudio Toniolo 1, 2 , Cristina Peggion 1, 2
Affiliation
|
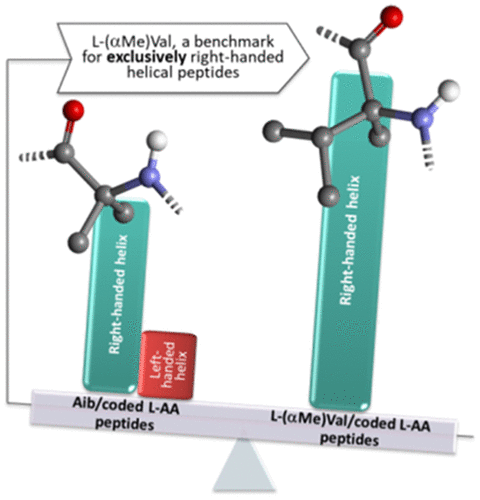
In synthetic peptides containing Gly and coded α-amino acids, one of the most common practices to enhance their helical extent is to incorporate a large number of l-Ala residues along with noncoded, strongly foldameric α-aminoisobutyric acid (Aib) units. Earlier studies have established that Aib-based peptides, with propensity for both the 310- and α-helices, have a tendency to form ordered three-dimensional structure that is much stronger than that exhibited by their l-Ala rich counterparts. However, the achiral nature of Aib induces an inherent, equal preference for the right- and left-handed helical conformations as found in Aib homopeptide stretches. This property poses challenges in the analysis of a model peptide helical conformation based on chirospectroscopic techniques like electronic circular dichroism (ECD), a very important tool for assigning secondary structures. To overcome such ambiguity, we have synthesized and investigated a thermally stable 14-mer peptide in which each of the Aib residues of our previously designed and reported analogue ABGY (where B stands for Aib) is replaced by Cα-methyl-l-valine (L-AMV). Analysis of the results described here from complementary ECD and 1H nuclear magnetic resonance spectroscopic techniques in a variety of environments firmly establishes that the L-AMV-containing peptide exhibits a significantly stronger preference compared to that of its Aib parent in terms of conferring α-helical character. Furthermore, being a chiral α-amino acid, L-AMV shows an intrinsic, extremely strong bias for a quite specific (right-handed) screw sense. These findings emphasize the relevance of L-AMV as a more appropriate unit for the design of right-handed α-helical peptide models that may be utilized as conformationally constrained scaffolds.
中文翻译:

Cα-甲基-l-缬氨酸:用于设计右手α-螺旋支架的首选α-氨基异丁酸
在含有 Gly 和编码的 α-氨基酸的合成肽中,增强其螺旋程度的最常见做法之一是将大量l -Ala 残基与非编码的、强折叠的 α-氨基异丁酸 (Aib) 单元结合起来。早期的研究已经确定,基于 Aib 的肽,具有 3 10 - 和 α-螺旋的倾向,有形成有序三维结构的趋势,比它们的l-阿拉富有的同行。然而,Aib 的非手性性质导致了对右手和左手螺旋构象的固有的、平等的偏好,如在 Aib 同源肽段中发现的。这种特性对基于手性光谱技术(如电子圆二色性 (ECD))的模型肽螺旋构象分析提出了挑战,电子圆二色性 (ECD) 是一种非常重要的二级结构分配工具。为了克服这种模糊性,我们已经合成并研究了热稳定的14-mer肽,其中每个的我们的先前设计并报告类似物ABGY(其中B代表AIB)被替换为C中的Aib取代残基的α -甲基-升-缬氨酸(L-AMV)。对此处描述的来自补充 ECD 和1的结果的分析各种环境中的 H 核磁共振光谱技术牢固地证明,在赋予 α-螺旋特征方面,含 L-AMV 的肽与其 Aib 亲本相比表现出明显更强的偏好。此外,作为一种手性 α-氨基酸,L-AMV 显示出对非常特定(右手)螺旋感的内在的、极强的偏见。这些发现强调了 L-AMV 作为设计右手α-螺旋肽模型更合适的单位的相关性,该模型可用作构象受限的支架。
更新日期:2021-09-14
中文翻译:

Cα-甲基-l-缬氨酸:用于设计右手α-螺旋支架的首选α-氨基异丁酸
在含有 Gly 和编码的 α-氨基酸的合成肽中,增强其螺旋程度的最常见做法之一是将大量l -Ala 残基与非编码的、强折叠的 α-氨基异丁酸 (Aib) 单元结合起来。早期的研究已经确定,基于 Aib 的肽,具有 3 10 - 和 α-螺旋的倾向,有形成有序三维结构的趋势,比它们的l-阿拉富有的同行。然而,Aib 的非手性性质导致了对右手和左手螺旋构象的固有的、平等的偏好,如在 Aib 同源肽段中发现的。这种特性对基于手性光谱技术(如电子圆二色性 (ECD))的模型肽螺旋构象分析提出了挑战,电子圆二色性 (ECD) 是一种非常重要的二级结构分配工具。为了克服这种模糊性,我们已经合成并研究了热稳定的14-mer肽,其中每个的我们的先前设计并报告类似物ABGY(其中B代表AIB)被替换为C中的Aib取代残基的α -甲基-升-缬氨酸(L-AMV)。对此处描述的来自补充 ECD 和1的结果的分析各种环境中的 H 核磁共振光谱技术牢固地证明,在赋予 α-螺旋特征方面,含 L-AMV 的肽与其 Aib 亲本相比表现出明显更强的偏好。此外,作为一种手性 α-氨基酸,L-AMV 显示出对非常特定(右手)螺旋感的内在的、极强的偏见。这些发现强调了 L-AMV 作为设计右手α-螺旋肽模型更合适的单位的相关性,该模型可用作构象受限的支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号