当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Human induced pluripotent stem cell-derived macrophages ameliorate liver fibrosis
STEM CELLS ( IF 4.0 ) Pub Date : 2021-08-30 , DOI: 10.1002/stem.3449 Somayeh Pouyanfard 1 , Nairika Meshgin 2 , Luisjesus S Cruz 1 , Karin Diggle 2 , Hamidreza Hashemi 3 , Timothy V Pham 4 , Manuel Fierro 1 , Pablo Tamayo 4 , Andrea Fanjul 5 , Tatiana Kisseleva 2 , Dan S Kaufman 1
STEM CELLS ( IF 4.0 ) Pub Date : 2021-08-30 , DOI: 10.1002/stem.3449 Somayeh Pouyanfard 1 , Nairika Meshgin 2 , Luisjesus S Cruz 1 , Karin Diggle 2 , Hamidreza Hashemi 3 , Timothy V Pham 4 , Manuel Fierro 1 , Pablo Tamayo 4 , Andrea Fanjul 5 , Tatiana Kisseleva 2 , Dan S Kaufman 1
Affiliation
|
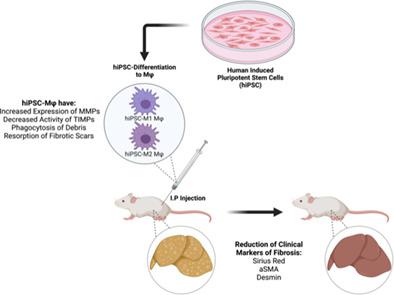
With an increasing number of patients with degenerative hepatic diseases, such as liver fibrosis, and a limited supply of donor organs, there is an unmet need for therapies that can repair or regenerate damaged liver tissue. Treatment with macrophages that are capable of phagocytosis and anti-inflammatory activities such as secretion of matrix metalloproteinases (MMPs) provide an attractive cellular therapy approach. Human induced pluripotent stem cells (iPSCs) are capable of efficiently generating a large-scale, homogenous population of human macrophages using fully defined feeder- and serum-free differentiation protocol. Human iPSC-macrophages exhibit classical surface cell markers and phagocytic activity similar to peripheral blood-derived macrophages. Moreover, gene and cytokine expression analysis reveal that these macrophages can be efficiently polarized to pro-inflammatory M1 or anti-inflammatory M2 phenotypes in presence of LPS + IFN-γ and IL-4 + IL-13, respectively. M1 macrophages express high level of CD80, TNF-α, and IL-6 while M2 macrophages show elevated expression of CD206, CCL17, and CCL22. Here, we demonstrate that treatment of liver fibrosis with both human iPSC-derived macrophage populations and especially M2 subtype significantly reduces fibrogenic gene expression and disease associated histological markers including Sirius Red, αSMA and desmin in immunodeficient Rag2−/−γc−/− mice model, making this approach a promising cell-based avenue to ameliorate fibrosis.
中文翻译:

人诱导多能干细胞来源的巨噬细胞改善肝纤维化
随着患有退行性肝病(例如肝纤维化)的患者数量不断增加,以及供体器官的供应有限,对能够修复或再生受损肝组织的疗法的需求尚未得到满足。使用具有吞噬作用和抗炎活性(例如分泌基质金属蛋白酶(MMP))的巨噬细胞进行治疗提供了一种有吸引力的细胞治疗方法。人类诱导多能干细胞 (iPSC) 能够使用完全定义的无饲养层和无血清分化方案,有效生成大规模、同质的人类巨噬细胞群。人 iPSC 巨噬细胞表现出与外周血来源的巨噬细胞类似的经典表面细胞标记和吞噬活性。此外,基因和细胞因子表达分析表明,在 LPS + IFN-γ 和 IL-4 + IL-13 存在的情况下,这些巨噬细胞可以分别有效地极化为促炎 M1 或抗炎 M2 表型。 M1 巨噬细胞表达高水平的 CD80、TNF-α 和 IL-6,而 M2 巨噬细胞则表达升高的 CD206、CCL17 和 CCL22。在这里,我们证明,在免疫缺陷 Rag2 −/− γc −/−小鼠模型中,用人 iPSC 衍生的巨噬细胞群,尤其是 M2 亚型治疗肝纤维化,可显着降低纤维化基因表达和疾病相关组织学标志物,包括天狼星红、αSMA 和结蛋白,使这种方法成为一种有前途的基于细胞的改善纤维化的途径。
更新日期:2021-08-30
中文翻译:

人诱导多能干细胞来源的巨噬细胞改善肝纤维化
随着患有退行性肝病(例如肝纤维化)的患者数量不断增加,以及供体器官的供应有限,对能够修复或再生受损肝组织的疗法的需求尚未得到满足。使用具有吞噬作用和抗炎活性(例如分泌基质金属蛋白酶(MMP))的巨噬细胞进行治疗提供了一种有吸引力的细胞治疗方法。人类诱导多能干细胞 (iPSC) 能够使用完全定义的无饲养层和无血清分化方案,有效生成大规模、同质的人类巨噬细胞群。人 iPSC 巨噬细胞表现出与外周血来源的巨噬细胞类似的经典表面细胞标记和吞噬活性。此外,基因和细胞因子表达分析表明,在 LPS + IFN-γ 和 IL-4 + IL-13 存在的情况下,这些巨噬细胞可以分别有效地极化为促炎 M1 或抗炎 M2 表型。 M1 巨噬细胞表达高水平的 CD80、TNF-α 和 IL-6,而 M2 巨噬细胞则表达升高的 CD206、CCL17 和 CCL22。在这里,我们证明,在免疫缺陷 Rag2 −/− γc −/−小鼠模型中,用人 iPSC 衍生的巨噬细胞群,尤其是 M2 亚型治疗肝纤维化,可显着降低纤维化基因表达和疾病相关组织学标志物,包括天狼星红、αSMA 和结蛋白,使这种方法成为一种有前途的基于细胞的改善纤维化的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号