Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2021-08-30 , DOI: 10.1016/j.yjmcc.2021.08.010 Dihan Zhu 1 , Yang Wang 1 , Miracle Thomas 1 , KeAsiah McLaughlin 1 , Babayewa Oguljahan 1 , Joshua Henderson 1 , Qinglin Yang 2 , Y Eugene Chen 3 , Dong Liu 4
|
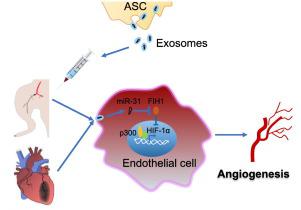
Our previous study has revealed that exosomes from adipose-derived stem cells (ASCs) promote angiogenesis in subcutaneously transplanted gels by delivery of microRNA-31 (miR-31) which targets factor inhibiting hypoxia-inducible factor-1 (FIH1) in recipient cells. Here we hypothesized that ASC exosomes alleviate ischemic diseases through miR-31/FIH1/hypoxia-inducible factor-1α (HIF-1α) signaling pathway. Exosomes from ASCs were characterized with nanoparticle tracking analysis, transmission electron microscopy, and immunoblotting analysis for exosomal markers. Results from immunoblotting and laser imaging of ischemic mouse hindlimb revealed that miR-31 enriched ASC exosomes inhibited FIH1 expression and enhanced the blood perfusion, respectively. These effects were impaired when using miR-31-depleted exosomes. Immunohistochemistry analysis showed that administration of exosomes resulted in a higher arteriole density and larger CD31+ area in ischemic hindlimb than miR-31-delpleted exosomes. Similarly, knockdown of miR-31 in exosomes reduced the effects of the exosomes on increasing ventricular fraction shortening and CD31+ area, and on decreasing infarct size. Exosomes promoted endothelial cell migration and tube formation. These changes were attenuated when miR-31 was depleted in the exosomes or when FIH1 was overexpressed in the endothelial cells. Furthermore, the results from immunocytochemistry, co-immunoprecipitation, and luciferase reporter assay demonstrated that the effects of exosomes on nuclear translocation, binding with co-activator p300, and activation of HIF-1α were decreased when miR-31 was depleted in the exosomes or FIH1 was overexpressed. Our findings provide evidence that exosomes from ASCs promote angiogenesis in both mouse ischemic hindlimb and heart through transport of miR-31 which targets FIH1 and therefore triggers HIF-1α transcriptional activation.
中文翻译:

来自脂肪干细胞的外泌体通过 microRNA-31/FIH1/HIF-1α 通路缓解心肌梗死
我们之前的研究表明,来自脂肪干细胞 (ASC) 的外泌体通过递送 microRNA-31 (miR-31) 促进皮下移植凝胶中的血管生成,microRNA-31 (miR-31) 靶向受体细胞中抑制缺氧诱导因子-1 (FIH1) 的因子。在这里,我们假设 ASC 外泌体通过 miR-31/FIH1/缺氧诱导因子-1α (HIF-1α) 信号通路缓解缺血性疾病。来自 ASCs 的外泌体通过纳米粒子追踪分析、透射电子显微镜和外泌体标志物的免疫印迹分析进行了表征。缺血性小鼠后肢的免疫印迹和激光成像结果显示,富含 miR-31 的 ASC 外泌体分别抑制 FIH1 表达和增强血液灌注。当使用 miR-31 耗尽的外泌体时,这些作用会受到损害。+缺血后肢的面积比 miR-31 耗尽的外泌体。类似地,外泌体中 miR-31 的敲低降低了外泌体对增加心室分数缩短和 CD31 +面积,并在减少梗塞面积。外泌体促进内皮细胞迁移和管形成。当 miR-31 在外泌体中耗尽或 FIH1 在内皮细胞中过度表达时,这些变化会减弱。此外,免疫细胞化学、免疫共沉淀和荧光素酶报告基因分析的结果表明,当 miR-31 在外泌体中耗尽或FIH1 过度表达。我们的研究结果提供了证据,表明来自 ASC 的外泌体通过转运靶向 FIH1 并因此触发 HIF-1α 转录激活的 miR-31 促进小鼠缺血后肢和心脏的血管生成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号