Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2021-08-30 , DOI: 10.1016/j.jcou.2021.101695 Lucia Veltri 1 , Roberta Amuso 1 , Paola Vitale 2 , Maria A. Chiacchio 3 , Cinzia Benincasa 4 , Bartolo Gabriele 1
|
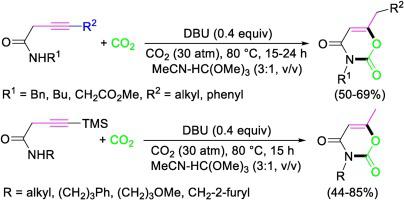
A new process for the utilization of carbon dioxide to give high value added 1,3-oxazine-2,4-diones is presented. It is based on the catalytic carboxylative heterocyclization of readily available 3-ynamides, using 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as organocatalyst. The reaction, which takes place under relatively mild conditions [80 °C for 15 h in a 3:1 mixture (v/v) of MeCN-HC(OMe)3, under 30 atm of CO2] proceeds through an ordered sequence of steps, involving substrate deprotonation by DBU, nucleophilic attack to CO2, 6-exo-dig cyclization, and isomerization, to give the final products in 44–85 % yields over 16 examples.
中文翻译:

通过 DBU 催化将二氧化碳掺入 3-ynamides 合成 1,3-oxazine-2,4-diones
提出了一种利用二氧化碳生产高附加值 1,3-恶嗪-2,4-二酮的新工艺。它基于容易获得的 3-炔酰胺的催化羧化杂环化,使用 1,8-二氮杂双环 [5.4.0] 十一碳-7-烯 (DBU) 作为有机催化剂。该反应在相对温和的条件下进行 [80 °C,在 3:1 的 MeCN-HC(OMe) 3混合物 (v/v) 中,在 30 个大气压的 CO 2下进行 15 小时]包括通过 DBU 进行底物去质子化、对 CO 2 的亲核攻击、6- exo - dig环化和异构化的步骤,在 16 个实例中以 44-85% 的产率得到最终产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号