Chinese Journal of Catalysis ( IF 15.7 ) Pub Date : 2021-08-30 , DOI: 10.1016/s1872-2067(21)63829-9 Jiayue Rong 1 , Zhenzhen Wang 1 , Jiaqi Lv 1 , Ming Fan 1 , Ruifeng Chong 1 , Zhixian Chang 1
|
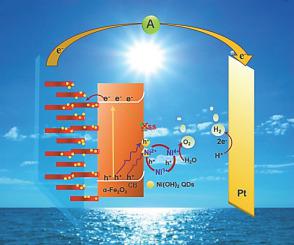
Depositing a cocatalyst has proven to be an important strategy for improving the photoelectrochemical (PEC) water-splitting efficiency of photoanodes. In this study, Ni(OH)2 quantum dots (Ni(OH)2 QDs) were deposited in situ onto an α-Fe2O3 photoanode via a chelation-mediated hydrolysis method. The photocurrent density of the Ni(OH)2 QDs/α-Fe2O3 photoanode reached 1.93 mA(cm−2 at 1.23 V vs. RHE, which is 3.5 times that of α-Fe2O3, and an onset potential with a negative shift of ca. 100 mV was achieved. More importantly, the Ni(OH)2 QDs exhibited excellent stability in maintaining PEC water oxidation at a high current density, which is attributed to the ultra-small crystalline size, allowing for the rapid acceptance of holes from α-Fe2O3 to Ni(OH)2 QDs, formation of active sites for water oxidation, and hole transfer from the active sites to water molecules. Further (photo)electrochemical analysis suggests that Ni(OH)2 QDs not only provide maximal active sites for water oxidation but also suppress charge recombination by passivating the surface states of α-Fe2O3, thereby significantly enhancing the water oxidation kinetics over the α-Fe2O3 surface.
中文翻译:

Ni(OH)2 量子点作为稳定助催化剂修饰的 α-Fe2O3 用于增强光电化学水分解
沉积助催化剂已被证明是提高光阳极光电化学(PEC)水分解效率的重要策略。在这项研究中,Ni(OH) 2量子点(Ni(OH) 2 QDs)通过螯合介导的水解方法原位沉积在α-Fe 2 O 3 光阳极上。Ni(OH) 2 QDs/α-Fe 2 O 3 光阳极的光电流密度达到1.93 mA(cm -2 at 1.23 V vs. RHE,是α-Fe 2 O 3 的3.5倍,起始电位负移约。达到了 100 mV。更重要的是,Ni(OH) 2 QDs 在高电流密度下保持 PEC 水氧化方面表现出优异的稳定性,这归因于超小的晶体尺寸,允许快速接受从 α-Fe 2 O 3到Ni(OH) 2 QD,水氧化活性位点的形成,以及空穴从活性位点转移到水分子。进一步的(光)电化学分析表明,Ni(OH) 2 QDs 不仅为水氧化提供了最大的活性位点,而且还通过钝化 α-Fe 2 O 3的表面态来抑制电荷复合,从而显着增强α-Fe 2 O 3表面上的水氧化动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号