Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Surfactant Concentration and Hydrophobicity on the Ordering of Water at a Silica Surface
Langmuir ( IF 3.7 ) Pub Date : 2021-08-29 , DOI: 10.1021/acs.langmuir.1c01731 Lirong Shi 1 , Janet R McMillan 2 , Decai Yu 2 , Xiaoyun Chen 2 , Christopher J Tucker 2 , Eric Wasserman 3 , Carol Mohler 2 , Zhan Chen 1
Langmuir ( IF 3.7 ) Pub Date : 2021-08-29 , DOI: 10.1021/acs.langmuir.1c01731 Lirong Shi 1 , Janet R McMillan 2 , Decai Yu 2 , Xiaoyun Chen 2 , Christopher J Tucker 2 , Eric Wasserman 3 , Carol Mohler 2 , Zhan Chen 1
Affiliation
|
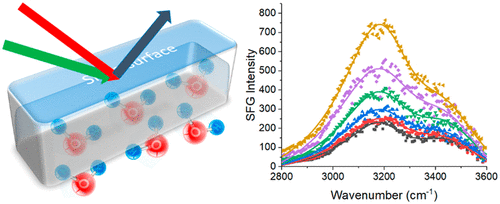
The performance of nonionic surfactants is mediated by the interfacial interactions at the solid–liquid interface. Here we applied sum frequency generation (SFG) vibrational spectroscopy to probe the molecular structure of the silica–nonionic surfactant solution interface in situ, supplemented by quartz crystal microbalance with dissipation monitoring (QCM-D) and molecular dynamics (MD) simulations. The combined studies elucidated the effects of nonionic surfactant solution concentration, surfactant composition, and rinsing on the silica–surfactant solution interfacial structure. The nonionic surfactants studied include ethylene-oxide (EO) and butylene oxide (BO) components with different ratios. It was found that the CH groups of the surfactants at the silica–surfactant solution interfaces are disordered, but the interfacial water molecules are ordered, generating strong SFG OH signals. Solutions with higher concentrations of surfactant lead to a slightly higher amount of adsorbed surfactant at the silica interface, resulting in more water molecules being ordered at the interface, or a higher ordering of water molecules at the interface, or both. MD simulation results indicated that the nonionic surface molecules preferentially adsorb onto silanol sites on silica. A surfactant with a higher EO/BO ratio leads to more water molecules being ordered and a higher degree of ordering of water molecules at the silica–surfactant solution interface, exhibiting stronger SFG OH signal, although less material is adsorbed according to the QCM-D data. A thin layer of surfactants remained on the silica surface after multiple water rinses. To the best of our knowledge, this is the first time the combined approaches of SFG, QCM-D and MD simulation techniques have been applied to study nonionic surfactants at the silica–solution interface, which enhances our understanding on the interfacial interactions between nonionic surfactants, water and silica. The knowledge obtained from this study can be helpful to design the optimal surfactant concentration and composition for future applications.
中文翻译:

表面活性剂浓度和疏水性对二氧化硅表面水的有序性的影响
非离子表面活性剂的性能由固液界面的界面相互作用介导。在这里,我们应用和频发生 (SFG) 振动光谱来原位探测二氧化硅-非离子表面活性剂溶液界面的分子结构,并辅以具有耗散监测 (QCM-D) 和分子动力学 (MD) 模拟的石英晶体微天平。联合研究阐明了非离子表面活性剂溶液浓度、表面活性剂组成和漂洗对二氧化硅-表面活性剂溶液界面结构的影响。研究的非离子表面活性剂包括不同比例的环氧乙烷 (EO) 和环氧丁烷 (BO) 组分。发现二氧化硅-表面活性剂溶液界面处表面活性剂的 CH 基团是无序的,但界面水分子是有序的,产生强烈的 SFG OH 信号。表面活性剂浓度较高的溶液会导致二氧化硅界面处吸附的表面活性剂数量略多,从而导致更多的水分子在界面上有序排列,或界面上的水分子排列更高,或两者兼而有之。MD 模拟结果表明非离子表面分子优先吸附到二氧化硅上的硅烷醇位点上。具有较高 EO/BO 比率的表面活性剂导致更多的水分子有序,并且在二氧化硅 - 表面活性剂溶液界面处水分子的有序程度更高,表现出更强的 SFG OH 信号,尽管根据 QCM-D 吸附的材料较少数据。在多次水冲洗后,表面活性剂薄层保留在二氧化硅表面上。据我们所知,这是首次将 SFG、QCM-D 和 MD 模拟技术的组合方法应用于研究二氧化硅 - 溶液界面的非离子表面活性剂,这增强了我们对非离子表面活性剂之间界面相互作用的理解、水和二氧化硅。从这项研究中获得的知识有助于为未来的应用设计最佳的表面活性剂浓度和组成。
更新日期:2021-09-14
中文翻译:

表面活性剂浓度和疏水性对二氧化硅表面水的有序性的影响
非离子表面活性剂的性能由固液界面的界面相互作用介导。在这里,我们应用和频发生 (SFG) 振动光谱来原位探测二氧化硅-非离子表面活性剂溶液界面的分子结构,并辅以具有耗散监测 (QCM-D) 和分子动力学 (MD) 模拟的石英晶体微天平。联合研究阐明了非离子表面活性剂溶液浓度、表面活性剂组成和漂洗对二氧化硅-表面活性剂溶液界面结构的影响。研究的非离子表面活性剂包括不同比例的环氧乙烷 (EO) 和环氧丁烷 (BO) 组分。发现二氧化硅-表面活性剂溶液界面处表面活性剂的 CH 基团是无序的,但界面水分子是有序的,产生强烈的 SFG OH 信号。表面活性剂浓度较高的溶液会导致二氧化硅界面处吸附的表面活性剂数量略多,从而导致更多的水分子在界面上有序排列,或界面上的水分子排列更高,或两者兼而有之。MD 模拟结果表明非离子表面分子优先吸附到二氧化硅上的硅烷醇位点上。具有较高 EO/BO 比率的表面活性剂导致更多的水分子有序,并且在二氧化硅 - 表面活性剂溶液界面处水分子的有序程度更高,表现出更强的 SFG OH 信号,尽管根据 QCM-D 吸附的材料较少数据。在多次水冲洗后,表面活性剂薄层保留在二氧化硅表面上。据我们所知,这是首次将 SFG、QCM-D 和 MD 模拟技术的组合方法应用于研究二氧化硅 - 溶液界面的非离子表面活性剂,这增强了我们对非离子表面活性剂之间界面相互作用的理解、水和二氧化硅。从这项研究中获得的知识有助于为未来的应用设计最佳的表面活性剂浓度和组成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号