Energy Storage Materials ( IF 18.9 ) Pub Date : 2021-08-30 , DOI: 10.1016/j.ensm.2021.08.037 Yanli Niu 1 , Xue Teng 1 , Shuaiqi Gong 1 , Xuan Liu 1 , Mingze Xu 1 , Zuofeng Chen 1
|
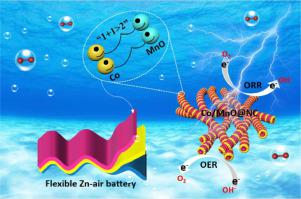
The highly active and robust bifunctional non-noble electrocatalysts for both oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) are urgently demanded for rechargeable metal-air batteries. Engineering heterointerfaces between metals and metal compounds is an attractive strategy for fabricating high-performance electrocatalysts. However, most reported metal/metal compound heterostructures were designed by partially converting the metal into corresponding metal compound on a one-metal basis. Herein, a facile “coordination construction-thermal decomposition” strategy is developed to construct Co/MnO heterointerface embedded in N-doped carbon nanowires by employing nitrilotriacetic acid as chelating agents to stabilize different metal ions and construct the nanowire structure. The in situ generated Co nanocrystals not only create highly conductive heterointerfaces with MnO, but also facilitate the formation of graphitic carbon as the conductive agent. The resultant Co/MnO@NC exhibits extraordinary bifunctional oxygen electrocatalytic activities and durability with a reversible oxygen overpotential of only 0.66 V. Density functional theory calculations reveal that the formation of Co/MnO heterostructure leads to optimized adsorption energy for oxygen-containing intermediates in OER and ORR. As the air-cathode, the assembled aqueous zinc-air battery (ZAB) can provide a remarkable peak power density of 146 mW cm−2 and an impressive discharge/charge stability for 400 h/600 cycles at 20 mA cm−2. The Co/MnO@NC-based quasi-solid-state ZAB also exhibits outstanding mechanical flexibility in addition to high battery performance. This strategy can be extended to prepare Fe/MnO@NC and Ni/MnO@NC electrocatalysts with other iron group elements, which paves a new way for development of efficient and robust electrocatalysts for energy conversion and storage.
中文翻译:

通过在多孔碳纳米线中连接铁族金属和氧化锰来促进柔性锌空气电池的氧电催化
可充电金属-空气电池迫切需要用于析氧反应(OER)和氧还原反应(ORR)的高活性和坚固的双功能非贵重电催化剂。在金属和金属化合物之间设计异质界面是制造高性能电催化剂的一种有吸引力的策略。然而,大多数报道的金属/金属化合物异质结构是通过在一种金属的基础上将金属部分转化为相应的金属化合物来设计的。在此,开发了一种简便的“配位构建-热分解”策略,通过使用次氮基三乙酸作为螯合剂来稳定不同金属离子并构建纳米线结构,从而构建嵌入N掺杂碳纳米线中的Co/MnO 异质界面。这原位生成的 Co 纳米晶体不仅可以与 MnO 形成高导电异质界面,还可以促进石墨碳作为导电剂的形成。所得的 Co/MnO@NC 表现出非凡的双功能氧电催化活性和耐久性,可逆氧过电位仅为 0.66 V。密度泛函理论计算表明,Co/MnO 异质结构的形成导致 OER 中含氧中间体的吸附能得到优化和 ORR。作为空气阴极,组装的水性锌空气电池 (ZAB) 可提供 146 mW cm -2的显着峰值功率密度和令人印象深刻的放电/充电稳定性,可在 20 mA cm -2 下进行 400 小时/600 次循环. 除了高电池性能外,基于 Co/MnO@NC 的准固态 ZAB 还表现出出色的机械柔韧性。该策略可以扩展到与其他铁族元素一起制备 Fe/MnO@NC 和 Ni/MnO@NC 电催化剂,为开发高效、稳健的能量转换和存储电催化剂铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号