Gastroenterology ( IF 25.7 ) Pub Date : 2021-08-30 , DOI: 10.1053/j.gastro.2021.08.045 William J Sandborn 1 , Larry C Mattheakis 2 , Nishit B Modi 2 , David Pugatch 3 , Brian Bressler 4 , Scott Lee 5 , Raj Bhandari 6 , Bittoo Kanwar 7 , Richard Shames 8 , Geert D'Haens 9 , Stefan Schreiber 10 , Silvio Danese 11 , Brian Feagan 12 , Rish K Pai 13 , David Y Liu 2 , Suneel Gupta 2
|
Background & Aims
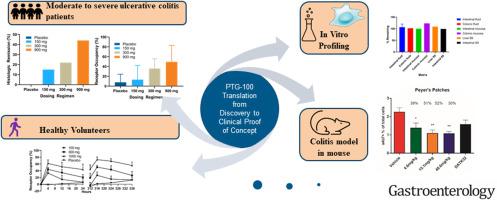
Oral therapies targeting the integrin α4β7 may offer unique advantages for the treatment of inflammatory bowel disease. We characterized the oral α4β7 antagonist peptide PTG-100 in preclinical models and established safety, pharmacokinetic/pharmacodynamic relationships, and efficacy in a phase 2a trial in patients with ulcerative colitis (UC).
Methods
In vitro studies measured binding properties of PTG-100. Mouse studies measured biomarkers and drug concentrations in blood and tissues. The phase 1 study involved healthy volunteers. In phase 2a, patients with moderate to severe active UC were randomized to receive PTG-100 (150, 300, or 900 mg) or placebo once daily for 12-weeks.
Results
PTG-100 potently and selectively blocks α4β7. Oral dosing of PTG-100 in mice showed high levels of target engagement and exposure in gut-associated lymphoid tissues. In healthy volunteers, PTG-100 showed dose-dependent increases in plasma exposure and blood target engagement. Although this phase 2a study initially did not meet the primary endpoint, a blinded reread of the endoscopy videos by a third party indicated clinical efficacy in conjunction with histologic remission at doses correlating with less than 100% receptor occupancy in peripheral blood.
Conclusions
PTG-100 showed local gastrointestinal tissue target engagement and inhibition of memory T-cell trafficking in mice. It was safe and well tolerated in phase 1 and 2 studies. Phase 2a data are consistent with biological and clinical response and showed a dose response reflecting similar activities in preclinical models and healthy individuals. These data suggest that local gut activity of an oral α4β7 integrin antagonist, distinct from full target engagement in blood, are important for efficacy and the treatment of UC. (ClinicalTrials.gov, Number NCT02895100; EudraCT, Number 2016-003452-75)
中文翻译:

PTG-100,一种口服 α4β7 拮抗剂肽:溃疡性结肠炎的临床前开发以及 1 期和 2a 期研究
背景与目标
针对整合素α4β7的口服疗法可能为炎症性肠病的治疗提供独特的优势。我们在临床前模型中对口服 α4β7 拮抗剂肽 PTG-100 进行了表征,并在溃疡性结肠炎 (UC) 患者的 2a 期试验中建立了安全性、药代动力学/药效学关系和疗效。
方法
体外研究测量了 PTG-100 的结合特性。小鼠研究测量了血液和组织中的生物标志物和药物浓度。第一阶段研究涉及健康志愿者。在第 2a 阶段,中度至重度活动性 UC 患者被随机接受 PTG-100(150、300 或 900 mg)或安慰剂,每天一次,持续 12 周。
结果
PTG-100 有效且选择性地阻断 α4β7。小鼠口服 PTG-100 在肠道相关淋巴组织中显示出高水平的靶点参与和暴露。在健康志愿者中,PTG-100 的血浆暴露和血液靶标参与度呈剂量依赖性增加。尽管这项 2a 期研究最初没有达到主要终点,但第三方对内窥镜视频的盲重重读表明,在与外周血中受体占有率低于 100% 相关的剂量下,具有临床疗效和组织学缓解。
结论
PTG-100 显示了小鼠局部胃肠道组织靶标参与和记忆 T 细胞运输的抑制。在 1 期和 2 期研究中,它是安全的且耐受性良好。 2a 期数据与生物学和临床反应一致,并显示剂量反应反映了临床前模型和健康个体中的类似活动。这些数据表明,口服 α4β7 整合素拮抗剂的局部肠道活性与血液中的完全靶点参与不同,对于 UC 的疗效和治疗非常重要。 (ClinicalTrials.gov,编号 NCT02895100;EudraCT,编号 2016-003452-75)











































 京公网安备 11010802027423号
京公网安备 11010802027423号