当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Study of the Reactions of 3-Bromopropenals with Anilines for the Synthesis of α-Bromo Enaminones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-08-27 , DOI: 10.1002/adsc.202100779 Sundaram Suresh, Prakash Bhimrao Patil, Pao-Hsing Yu, Chia-Chi Fang, Yin-Zhi Weng, Veerababurao Kavala, Ching-Fa Yao
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-08-27 , DOI: 10.1002/adsc.202100779 Sundaram Suresh, Prakash Bhimrao Patil, Pao-Hsing Yu, Chia-Chi Fang, Yin-Zhi Weng, Veerababurao Kavala, Ching-Fa Yao
|
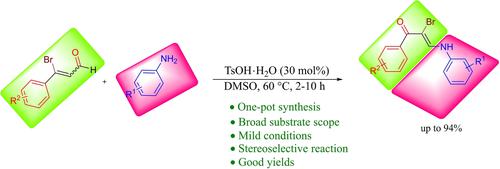
A one-pot strategy for the synthesis of α-bromo enaminones is reported. The reactions proceed via the p-toluenesulfonic acid monohydrate (TsOH ⋅ H2O) catalyzed reactions of 3-bromopropenals with anilines in dimethyl sulfoxide (DMSO) and does not require an external brominating agent. The chemoselective 1,2-addition was accomplished by employing aniline with a sterically hindered electron-withdrawing group attached at the ortho-position. In addition, the reactions involving other aniline derivatives as nucleophiles have resulted in minor yields of 1,4-addition product. The 3-bromopropenals showed a diverse range of reactivities with aniline derivatives.
中文翻译:

3-溴丙烯醛与苯胺反应合成α-溴烯胺酮的研究
报道了一种用于合成α-溴烯胺酮的一锅法。反应进行经由所述p -甲苯磺酸一水合物(加入TsOH⋅ħ 2 O)与在二甲亚砜(DMSO),并且不需要外部溴化剂苯胺3- bromopropenals催化的反应。化学选择性 1,2-加成是通过使用在邻位连接有位阻吸电子基团的苯胺来完成的。此外,涉及其他苯胺衍生物作为亲核试剂的反应导致 1,4-加成产物的产量很小。3-溴丙烯醛与苯胺衍生物表现出不同范围的反应性。
更新日期:2021-08-27
中文翻译:

3-溴丙烯醛与苯胺反应合成α-溴烯胺酮的研究
报道了一种用于合成α-溴烯胺酮的一锅法。反应进行经由所述p -甲苯磺酸一水合物(加入TsOH⋅ħ 2 O)与在二甲亚砜(DMSO),并且不需要外部溴化剂苯胺3- bromopropenals催化的反应。化学选择性 1,2-加成是通过使用在邻位连接有位阻吸电子基团的苯胺来完成的。此外,涉及其他苯胺衍生物作为亲核试剂的反应导致 1,4-加成产物的产量很小。3-溴丙烯醛与苯胺衍生物表现出不同范围的反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号