International Journal of Pharmaceutics ( IF 5.3 ) Pub Date : 2021-08-28 , DOI: 10.1016/j.ijpharm.2021.121057 Khalid M El-Say 1 , Tarek A Ahmed 1 , Arwa H Aljefri 2 , Hossam S El-Sawy 3 , Reza Fassihi 4 , Magid Abou-Gharbia 4
|
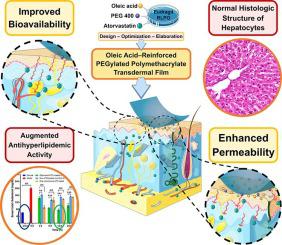
To enhance the poor bioavailability and extensive liver metabolism of atorvastatin calcium (ATC), we have developed an oleic acid–reinforced PEGylated polymethacrylate (OLA–PEG–E-RLPO) transdermal film as a convenient and alternative delivery system. The effect of varying levels of Eudragit RLPO, PEG 400, and oleic acid on the target product profile was optimized through Quality by Design (QbD) approach. The ATC–loaded OLA–PEG–E-RLPO transdermal films were evaluated in ex-vivo experiments using full thickness skin, utilizing Franz cell studies, and undergone in-vivo pharmacokinetics/pharmacodynamics (PK/PD) assessment, using poloxamer-induced dyslipidemic Sprague-Dawley rats. At 2 and 12 h, the optimized ATC films with a thickness of 0.79 mm showed permeation of 37.34% and 97.23% into the receptor compartment, respectively. Steady-state flux was 0.172 mg/cm2h, with 7.01 × 10−4 cm/h permeability coefficient, and 0.713 × 10−3 cm2/h diffusion coefficient. In-vivo PK results indicated that the absorption profiles (AUC0-∞) of the optimized film in pre-treated group of animals were 8.6-fold and 2.8-fold greater than controls pre-treated with non-PEGylated non-oleic acid film and orally administered ATC, respectively. PD assessment of the lipid panel indicated that the lipid profile of the optimized film pre-treated group reached normal levels after 12 h, along with the significant enhancement over the non–PEGylated non-oleic acid film and the oral marketed tablet groups. The histopathological findings revealed near-normal hepatocyte structure for the optimized film pre-treated animal group. Our results further indicate that transdermal delivery films based on an optimized ATC–loaded OLA–PEG–E-RLPO were successfully developed and their assessment in both ex-vivo and in-vivo suggests enhanced permeability and improvement in bioavailability and antidyslipidemic activity of ATC. This approach can provide several advantages, especially during chronic administration of ATC, including improvement in patient compliance, therapeutic benefits, bioavailability, and feasibility for commercialization and as a platform for other drug classes.
中文翻译:

具有增强的阿托伐他汀抗血脂异常活性和生物利用度的油酸增强聚甲基丙烯酸酯透皮膜:体外/体内机制分析
为了提高阿托伐他汀钙 (ATC) 较差的生物利用度和广泛的肝脏代谢,我们开发了一种油酸增强的聚甲基丙烯酸酯 (OLA-PEG-E-RLPO) 透皮薄膜作为一种方便的替代递送系统。通过质量源于设计 (QbD) 方法优化了不同水平的 Eudragit RLPO、PEG 400 和油酸对目标产品特征的影响。在体外实验中使用全厚度皮肤、利用 Franz 细胞研究评估了负载 ATC 的 OLA-PEG-E-RLPO 透皮薄膜,并在体内进行了药代动力学/药效学 (PK/PD) 评估,使用泊洛沙姆诱导的血脂异常 Sprague-Dawley 大鼠。在 2 小时和 12 小时时,厚度为 0.79 mm 的优化 ATC 膜分别显示出 37.34% 和 97.23% 的渗透率。稳态通量为0.172 mg/cm 2 h,渗透系数为7.01×10 -4 cm/h,扩散系数为0.713×10 -3 cm 2 /h。体内PK 结果表明吸收曲线 (AUC 0-∞) 在预处理组动物中优化膜的 ) 分别比用非聚乙二醇化非油酸膜预处理和口服 ATC 预处理的对照组高 8.6 倍和 2.8 倍。脂质组的 PD 评估表明,优化的薄膜预处理组的脂质谱在 12 小时后达到正常水平,同时显着增强了非聚乙二醇化非油酸薄膜和口服上市片剂组。组织病理学结果显示优化的薄膜预处理动物组的肝细胞结构接近正常。我们的结果进一步表明,基于优化的 ATC 负载 OLA-PEG-E-RLPO 的透皮给药薄膜已成功开发,并在体外和体内评估表明 ATC 的渗透性增强和生物利用度和抗血脂异常活性的改善。这种方法可以提供多种优势,尤其是在 ATC 的长期给药期间,包括提高患者依从性、治疗益处、生物利用度以及商业化和作为其他药物类别的平台的可行性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号