当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the Mechanism of Aerobic Alcohol Oxidation by a Cu/pytl-β-Cyclodextrin/TEMPO Catalytic System under Air in Neat Water
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2021-08-28 , DOI: 10.1021/acs.inorgchem.1c01504 Chunmei Feng 1 , Lin Cheng 1 , Huiyan Ma 1 , Lisha Ma 1 , Qi Wu 2 , Jucai Yang 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2021-08-28 , DOI: 10.1021/acs.inorgchem.1c01504 Chunmei Feng 1 , Lin Cheng 1 , Huiyan Ma 1 , Lisha Ma 1 , Qi Wu 2 , Jucai Yang 1
Affiliation
|
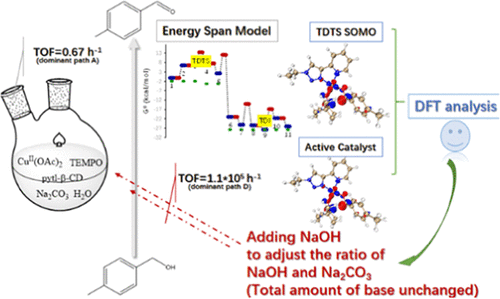
The mechanism for the oxidation of p-tolylmethanol to p-tolualdehyde catalyzed by a Cu/pytl-β-cyclodextrin/TEMPO (TEMPO = 2,2,6,6-tetramethylpiperidinyl-1-oxy) catalytic system under air in neat water is fully investigated by density functional theory (DFT). Four possible pathways (paths A → D) are presented. The calculated TOF = 0.67 h–1 for path A is consistent with the experimental TOF = 1.9 h–1 but much lower than that for path D (TOF = 1.1 × 105 h–1). The results demonstrate that path A is the dominant pathway under the optimal experimental conditions, even though path D is more kinetically favorable. This is because the concentration of precatalyst 11 [(pytl-β-CD)CuII(OH)] in path D is too low to start path D, so p-tolylmethanol oxidation can only proceed via path A. This finding implies that the relative concentration of precatalysts in a one-pot synthesis experiment plays a vital role in the aerobic alcohol oxidation reaction. Based on this finding, we speculate that the direct use of the presynthesized precatalyst 11 or addition of an appropriate amount of NaOH to the reaction solution, but with the total amount of the base added unchanged, is a good way to improve its catalytic activity. Meanwhile, the solvent water was not found to directly participate in the catalytic active sites for the oxidation of alcohols but rather inhibited it by forming the hydrogen-bonded network.
中文翻译:

Cu/pytl-β-环糊精/TEMPO催化体系在纯净水中在空气中氧化有氧醇的机理
用于氧化的机构p -tolylmethanol到p -tolualdehyde由铜/ pytl-β环糊精催化/ TEMPO(TEMPO = 2,2,6,6-四甲基-1-氧基)在纯净水在空气下的催化体系是通过密度泛函理论(DFT)进行了全面研究。介绍了四种可能的路径(路径 A → D)。路径 A的计算 TOF = 0.67 h –1与实验 TOF = 1.9 h –1一致,但远低于路径 D(TOF = 1.1 × 10 5 h –1)。结果表明,路径 A 是最佳实验条件下的主要路径,即使路径 D 在动力学上更有利。这是因为前催化剂11的浓度[(pytl-β-CD)Cu II (OH)] 在路径 D 中太低而无法开始路径 D,因此对甲苯基甲醇氧化只能通过路径 A 进行。这一发现意味着一个-锅合成实验在好氧醇氧化反应中起着至关重要的作用。基于这一发现,我们推测直接使用预合成的预催化剂11或向反应液中加入适量的NaOH,但加入的碱总量不变,是提高其催化活性的好方法。同时,没有发现溶剂水直接参与醇氧化的催化活性位点,而是通过形成氢键网络来抑制它。
更新日期:2021-09-20
中文翻译:

Cu/pytl-β-环糊精/TEMPO催化体系在纯净水中在空气中氧化有氧醇的机理
用于氧化的机构p -tolylmethanol到p -tolualdehyde由铜/ pytl-β环糊精催化/ TEMPO(TEMPO = 2,2,6,6-四甲基-1-氧基)在纯净水在空气下的催化体系是通过密度泛函理论(DFT)进行了全面研究。介绍了四种可能的路径(路径 A → D)。路径 A的计算 TOF = 0.67 h –1与实验 TOF = 1.9 h –1一致,但远低于路径 D(TOF = 1.1 × 10 5 h –1)。结果表明,路径 A 是最佳实验条件下的主要路径,即使路径 D 在动力学上更有利。这是因为前催化剂11的浓度[(pytl-β-CD)Cu II (OH)] 在路径 D 中太低而无法开始路径 D,因此对甲苯基甲醇氧化只能通过路径 A 进行。这一发现意味着一个-锅合成实验在好氧醇氧化反应中起着至关重要的作用。基于这一发现,我们推测直接使用预合成的预催化剂11或向反应液中加入适量的NaOH,但加入的碱总量不变,是提高其催化活性的好方法。同时,没有发现溶剂水直接参与醇氧化的催化活性位点,而是通过形成氢键网络来抑制它。



























 京公网安备 11010802027423号
京公网安备 11010802027423号