Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-08-28 , DOI: 10.1016/j.bioorg.2021.105301 Martin Krátký 1 , Quynh Anh Vu 1 , Šárka Štěpánková 2 , Annalisa Maruca 3 , Tiago Barros Silva 4 , Martin Ambrož 5 , Václav Pflégr 1 , Roberta Rocca 6 , Katarína Svrčková 2 , Stefano Alcaro 3 , Fernanda Borges 4 , Jarmila Vinšová 1
|
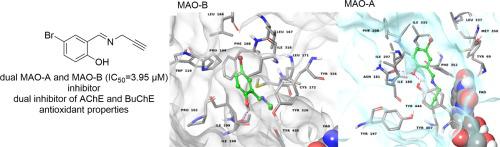
A combination of several pharmacophores in one molecule has been successfully used for multi-target-directed ligands (MTDL) design. New propargylamine substituted derivatives combined with salicylic and cinnamic scaffolds were designed and synthesized as potential cholinesterases and monoamine oxidases (MAOs) inhibitors. They were evaluated in vitro for inhibition of acetyl- (AChE) and butyrylcholinesterase (BuChE) using Ellman’s method. All the compounds act as dual inhibitors. Most of the derivatives are stronger inhibitors of AChE, the best activity showed 5-bromo-N-(prop-2-yn-1-yl)salicylamide 1e (IC50 = 8.05 µM). Carbamates (4-bromo-2-[(prop-2-yn-1-yl)carbamoyl]phenyl ethyl(methyl)carbamate 2d and 2,4-dibromo-6-[(prop-2-yn-1-yl)carbamoyl]phenyl ethyl(methyl)carbamate 2e were selective and the most active for BuChE (25.10 and 26.09 µM). 4-Bromo-2-[(prop-2-yn-1-ylimino)methyl]phenol 4a was the most potent inhibitor of MAOs (IC50 of 3.95 and ≈10 µM for MAO-B and MAO-A, respectively) along with a balanced inhibition of both cholinesterases being a real MTDL. The mechanism of action was proposed, and binding modes of the hits were studied by molecular docking on human enzymes. Some of the derivatives also exhibited antioxidant properties. In silico prediction of physicochemical parameters affirm that the molecules would be active after oral administration and able to reach brain tissue.
中文翻译:

基于炔丙胺的胆碱酯酶和单胺氧化酶抑制剂:合成、生物学评价和对接研究
一个分子中几种药效团的组合已成功用于多靶标配体 (MTDL) 设计。新的炔丙胺取代衍生物与水杨酸和肉桂酸支架结合被设计和合成为潜在的胆碱酯酶和单胺氧化酶 (MAO) 抑制剂。使用 Ellman 方法在 体外评估了它们对乙酰基 (AChE) 和丁酰胆碱酯酶 (BuChE) 的抑制作用。所有化合物均作为双重抑制剂。大多数衍生物是更强的 AChE 抑制剂,最佳活性显示 5-溴-N -(prop-2-yn-1-yl) 水杨酰胺1e (IC 50 = 8.05 µM)。氨基甲酸酯(4-溴-2-[(丙-2-炔-1-基)氨基甲酰基]苯基乙基(甲基)氨基甲酸酯2d和 2,4-dibromo-6-[(prop-2-yn-1-yl)carbamoyl] 苯基乙基(甲基)氨基甲酸酯2e对 BuChE 具有选择性且最活跃(25.10 和 26.09 µM)。4-Bromo-2-[(prop-2-yn-1-ylimino)methyl]phenol 4a是最有效的 MAO 抑制剂(MAO-B 和 MAO-A 的IC 50 分别为 3.95 和 ≈10 µM)。两种胆碱酯酶的平衡抑制是真正的 MTDL。提出了作用机制,并通过与人类酶的分子对接研究了命中的结合模式。一些衍生物还表现出抗氧化特性。在 计算机芯片上的物理化学参数的预测申明,分子将是口服给药后活性和能够到达脑组织。











































 京公网安备 11010802027423号
京公网安备 11010802027423号