当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oligonucleotide-Functionalized Gold Nanoparticles for Synchronous Telomerase Inhibition, Radiosensitization, and Delivery of Theranostic Radionuclides
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-08-27 , DOI: 10.1021/acs.molpharmaceut.1c00442 Bas M Bavelaar 1 , Lei Song 1 , Mark R Jackson 2 , Sarah Able 1 , Ole Tietz 1 , Irini Skaripa-Koukelli 1 , Philip A Waghorn 3 , Martin R Gill 1 , Robert C Carlisle 4 , Madalena Tarsounas 1 , Katherine A Vallis 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-08-27 , DOI: 10.1021/acs.molpharmaceut.1c00442 Bas M Bavelaar 1 , Lei Song 1 , Mark R Jackson 2 , Sarah Able 1 , Ole Tietz 1 , Irini Skaripa-Koukelli 1 , Philip A Waghorn 3 , Martin R Gill 1 , Robert C Carlisle 4 , Madalena Tarsounas 1 , Katherine A Vallis 1
Affiliation
|
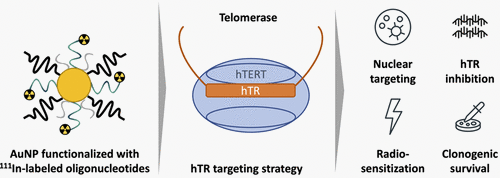
Telomerase represents an attractive target in oncology as it is expressed in cancer but not in normal tissues. The oligonucleotide inhibitors of telomerase represent a promising anticancer strategy, although poor cellular uptake can restrict their efficacy. In this study, gold nanoparticles (AuNPs) were used to enhance oligonucleotide uptake. “match” oligonucleotides complementary to the telomerase RNA template subunit (hTR) and “scramble” (control) oligonucleotides were conjugated to diethylenetriamine pentaacetate (DTPA) for 111In-labeling. AuNPs (15.5 nm) were decorated with a monofunctional layer of oligonucleotides (ON–AuNP) or a multifunctional layer of oligonucleotides, PEG(polethylene glycol)800-SH (to reduce AuNP aggregation) and the cell-penetrating peptide Tat (ON–AuNP–Tat). Match–AuNP enhanced the cellular uptake of radiolabeled oligonucleotides while retaining the ability to inhibit telomerase activity. The addition of Tat to AuNPs increased nuclear localization. 111In–Match–AuNP–Tat induced DNA double-strand breaks and caused a dose-dependent reduction in clonogenic survival of telomerase-positive cells but not telomerase-negative cells. hTR inhibition has been reported to sensitize cancer cells to ionizing radiation, and 111In–Match–AuNP–Tat therefore holds promise as a vector for delivery of radionuclides into cancer cells while simultaneously sensitizing them to the effects of the emitted radiation.
中文翻译:

用于同步端粒酶抑制、放射增敏和治疗诊断放射性核素递送的寡核苷酸功能化金纳米颗粒
端粒酶是肿瘤学中一个有吸引力的靶点,因为它在癌症中表达,但在正常组织中不表达。端粒酶寡核苷酸抑制剂代表了一种有前途的抗癌策略,尽管细胞摄取不良会限制其功效。在这项研究中,金纳米粒子 (AuNP) 用于增强寡核苷酸的吸收。与端粒酶 RNA 模板亚基 (hTR) 互补的“匹配”寡核苷酸和“乱序”(对照)寡核苷酸与二乙烯三胺五乙酸盐 (DTPA) 缀合以进行111 In 标记。 AuNP (15.5 nm) 装饰有单功能寡核苷酸层 (ON–AuNP) 或多功能寡核苷酸层 PEG(聚乙二醇)800-SH(以减少 AuNP 聚集)和细胞穿透肽 Tat (ON–AuNP) –达)。 Match-AuNP 增强了细胞对放射性标记寡核苷酸的摄取,同时保留了抑制端粒酶活性的能力。在 AuNP 中添加 Tat 可以增加核定位。 111 In-Match-AuNP-Tat 可诱导 DNA 双链断裂,并导致端粒酶阳性细胞(而非端粒酶阴性细胞)的克隆形成存活率出现剂量依赖性下降。据报道,hTR 抑制可使癌细胞对电离辐射敏感,因此111 In-Match-AuNP-Tat 有希望作为将放射性核素递送到癌细胞中的载体,同时使癌细胞对所发射的辐射的影响敏感。
更新日期:2021-10-04
中文翻译:

用于同步端粒酶抑制、放射增敏和治疗诊断放射性核素递送的寡核苷酸功能化金纳米颗粒
端粒酶是肿瘤学中一个有吸引力的靶点,因为它在癌症中表达,但在正常组织中不表达。端粒酶寡核苷酸抑制剂代表了一种有前途的抗癌策略,尽管细胞摄取不良会限制其功效。在这项研究中,金纳米粒子 (AuNP) 用于增强寡核苷酸的吸收。与端粒酶 RNA 模板亚基 (hTR) 互补的“匹配”寡核苷酸和“乱序”(对照)寡核苷酸与二乙烯三胺五乙酸盐 (DTPA) 缀合以进行111 In 标记。 AuNP (15.5 nm) 装饰有单功能寡核苷酸层 (ON–AuNP) 或多功能寡核苷酸层 PEG(聚乙二醇)800-SH(以减少 AuNP 聚集)和细胞穿透肽 Tat (ON–AuNP) –达)。 Match-AuNP 增强了细胞对放射性标记寡核苷酸的摄取,同时保留了抑制端粒酶活性的能力。在 AuNP 中添加 Tat 可以增加核定位。 111 In-Match-AuNP-Tat 可诱导 DNA 双链断裂,并导致端粒酶阳性细胞(而非端粒酶阴性细胞)的克隆形成存活率出现剂量依赖性下降。据报道,hTR 抑制可使癌细胞对电离辐射敏感,因此111 In-Match-AuNP-Tat 有希望作为将放射性核素递送到癌细胞中的载体,同时使癌细胞对所发射的辐射的影响敏感。









































 京公网安备 11010802027423号
京公网安备 11010802027423号