Synlett ( IF 1.7 ) Pub Date : 2021-08-26 , DOI: 10.1055/s-0040-1719829 Tianning Diao , Qiao Lin , Gregory Dawson
|
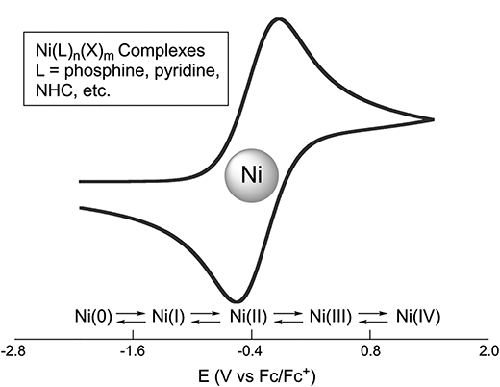
Nickel-catalyzed cross-coupling and photoredox catalytic reactions has found widespread utilities in organic synthesis. Redox processes are key intermediate steps in many catalytic cycles. As a result, it is pertinent to measure and document the redox potentials of various nickel species as precatalysts, catalysts, and intermediates. The redox potentials of a transition-metal complex are governed by its oxidation state, ligand, and the solvent environment. This article tabulates experimentally measured redox potentials of nickel complexes supported on common ligands under various conditions. This review article serves as a versatile tool to help synthetic organic and organometallic chemists evaluate the feasibility and kinetics of redox events occurring at the nickel center, when designing catalytic reactions and preparing nickel complexes.
1 Introduction
1.1 Scope
1.2 Measurement of Formal Redox Potentials
1.3 Redox Potentials in Nonaqueous Solution
2 Redox Potentials of Nickel Complexes
2.1 Redox Potentials of (Phosphine)Ni Complexes
2.2 Redox Potentials of (Nitrogen)Ni Complexes
2.3 Redox Potentials of (NHC)Ni Complexes
中文翻译:

镍配合物的实验电化学电位
镍催化的交叉偶联和光氧化还原催化反应已在有机合成中得到广泛应用。氧化还原过程是许多催化循环中的关键中间步骤。因此,有必要测量和记录作为前催化剂、催化剂和中间体的各种镍物种的氧化还原电位。过渡金属配合物的氧化还原电位受其氧化态、配体和溶剂环境的控制。本文列出了在各种条件下由常见配体支持的镍配合物的实验测量氧化还原电位。在设计催化反应和制备镍配合物时,这篇综述文章可作为一种通用工具,帮助合成有机和有机金属化学家评估发生在镍中心的氧化还原事件的可行性和动力学。
1 简介
1.1 范围
1.2 正式氧化还原电位的测量
1.3 非水溶液中的氧化还原电位
2 镍配合物的氧化还原电位
2.1 (Phosphine)Ni配合物的氧化还原电位
2.2 (氮)Ni配合物的氧化还原电位
2.3 (NHC)Ni配合物的氧化还原电位











































 京公网安备 11010802027423号
京公网安备 11010802027423号