当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Indium Catalyzed Sequential Regioselective Remote C−H Indolylation and Rearrangement Reaction of Peroxyoxindole
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-08-26 , DOI: 10.1002/adsc.202100793 Moseen A. Shaikh 1 , Akash S. Ubale 1 , Boopathy Gnanaprakasam 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-08-26 , DOI: 10.1002/adsc.202100793 Moseen A. Shaikh 1 , Akash S. Ubale 1 , Boopathy Gnanaprakasam 1
Affiliation
|
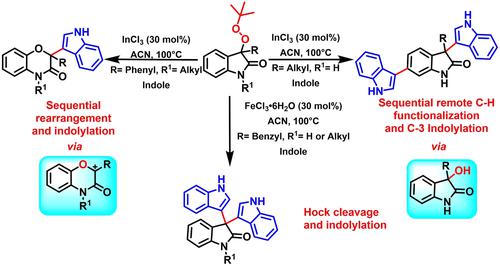
Indium-catalyzed sequential remote C−H functionalization (C-6 position) and C3-indolylation of peroxyoxindole using indole is described for the synthesis of terindolinone derivatives. Whereas, N-substituted 3-phenyl peroxyoxindole derivatives undergoes consecutive skeletal rearrangement to generate transient carbocation, which has been trapped with indole nucleophile to generate 2-(1H-indol-3-yl)-4-alkyl-benzo[b][1,4]oxazin-3(4H)-one derivatives. In contrast with Indium (III) Chloride, FeCl3 ⋅ 6H2O facilitates oxidative cleavage of the peroxyoxindole (Hock cleavage) and further reaction with indole to afford biologically important trisindoline derivatives. A plausible mechanism has been proposed for these reactions with experimental evidences.
中文翻译:

铟催化过氧化吲哚的顺序区域选择性远程C-H吲哚化和重排反应
描述了使用吲哚对过氧吲哚进行铟催化的连续远程 CH 官能化(C-6 位)和 C3-吲哚化,用于合成特吲哚啉酮衍生物。然而,N-取代的3-苯基过氧吲哚衍生物经历连续的骨架重排以产生瞬时碳正离子,其被吲哚亲核试剂捕获以产生2-(1 H-吲哚-3-基)-4-烷基-苯并[ b ][ 1,4] oxazin-3(4 H )-one 衍生物。与氯化铟 (III) 相比,FeCl 3 ⋅ 6H 2O 促进过氧吲哚的氧化裂解(Hock 裂解)并进一步与吲哚反应以提供具有生物学重要意义的三吲哚衍生物。已经为这些反应提出了一种可能的机制,并有实验证据。
更新日期:2021-08-26
中文翻译:

铟催化过氧化吲哚的顺序区域选择性远程C-H吲哚化和重排反应
描述了使用吲哚对过氧吲哚进行铟催化的连续远程 CH 官能化(C-6 位)和 C3-吲哚化,用于合成特吲哚啉酮衍生物。然而,N-取代的3-苯基过氧吲哚衍生物经历连续的骨架重排以产生瞬时碳正离子,其被吲哚亲核试剂捕获以产生2-(1 H-吲哚-3-基)-4-烷基-苯并[ b ][ 1,4] oxazin-3(4 H )-one 衍生物。与氯化铟 (III) 相比,FeCl 3 ⋅ 6H 2O 促进过氧吲哚的氧化裂解(Hock 裂解)并进一步与吲哚反应以提供具有生物学重要意义的三吲哚衍生物。已经为这些反应提出了一种可能的机制,并有实验证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号