当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Confinement-Controlled, Either syn- or anti-Selective Catalytic Asymmetric Mukaiyama Aldolizations of Propionaldehyde Enolsilanes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-08-26 , DOI: 10.1021/jacs.1c07447 Tynchtyk Amatov 1 , Nobuya Tsuji 2 , Rajat Maji 1 , Lucas Schreyer 1 , Hui Zhou 1 , Markus Leutzsch 1 , Benjamin List 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-08-26 , DOI: 10.1021/jacs.1c07447 Tynchtyk Amatov 1 , Nobuya Tsuji 2 , Rajat Maji 1 , Lucas Schreyer 1 , Hui Zhou 1 , Markus Leutzsch 1 , Benjamin List 1, 2
Affiliation
|
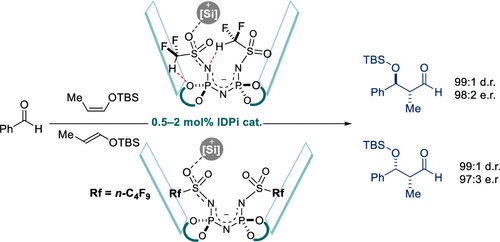
Protected aldols (i.e., true aldols derived from aldehydes) with either syn- or anti- stereochemistry are versatile intermediates in many oligopropionate syntheses. Traditional stereoselective approaches to such aldols typically require several nonstrategic operations. Here we report two highly enantioselective and diastereoselective catalytic Mukaiyama aldol reactions of the TBS- or TES- enolsilanes of propionaldehyde with aromatic aldehydes. Our reactions directly deliver valuable silyl protected propionaldehyde aldols in a catalyst controlled manner, either as syn- or anti- isomer. We have identified a privileged IDPi catalyst motif that is tailored for controlling these aldolizations with exceptional selectivities. We demonstrate how a single atom modification in the inner core of the IDPi catalyst, replacing a CF3-group with a CF2H-group, leads to a dramatic switch in enantiofacial differentiation of the aldehyde. The origin of this remarkable effect was attributed to tightening of the catalytic cavity via unconventional C–H hydrogen bonding of the CF2H group.
中文翻译:

丙醛烯醇硅烷的限制控制、顺式或反选择性催化不对称 Mukaiyama 醛醇化
具有顺式或反式立体化学的受保护醛醇(即来自醛的真正醛醇)是许多低聚丙酸酯合成中的通用中间体。这种醛醇的传统立体选择性方法通常需要几个非战略性操作。在这里,我们报告了丙醛的 TBS-或 TES-烯醇硅烷与芳香醛的两种高度对映选择性和非对映选择性催化 Mukaiyama 羟醛反应。我们的反应以催化剂控制的方式直接提供有价值的甲硅烷基保护的丙醛醛醇,无论是合成还是反异构体。我们已经确定了一种特权 IDPi 催化剂基序,该基序专为控制这些醛醇化反应而设计,具有出色的选择性。我们展示了 IDPi 催化剂内核中的单原子修饰,用CF 2 H 基团替换 CF 3基团,如何导致醛的对映面分化发生戏剧性的转变。这种显着效果的起源归因于通过 CF 2 H 基团的非常规 C-H 氢键使催化腔变紧。
更新日期:2021-09-15
中文翻译:

丙醛烯醇硅烷的限制控制、顺式或反选择性催化不对称 Mukaiyama 醛醇化
具有顺式或反式立体化学的受保护醛醇(即来自醛的真正醛醇)是许多低聚丙酸酯合成中的通用中间体。这种醛醇的传统立体选择性方法通常需要几个非战略性操作。在这里,我们报告了丙醛的 TBS-或 TES-烯醇硅烷与芳香醛的两种高度对映选择性和非对映选择性催化 Mukaiyama 羟醛反应。我们的反应以催化剂控制的方式直接提供有价值的甲硅烷基保护的丙醛醛醇,无论是合成还是反异构体。我们已经确定了一种特权 IDPi 催化剂基序,该基序专为控制这些醛醇化反应而设计,具有出色的选择性。我们展示了 IDPi 催化剂内核中的单原子修饰,用CF 2 H 基团替换 CF 3基团,如何导致醛的对映面分化发生戏剧性的转变。这种显着效果的起源归因于通过 CF 2 H 基团的非常规 C-H 氢键使催化腔变紧。











































 京公网安备 11010802027423号
京公网安备 11010802027423号