当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid 2H NMR Transverse Relaxation of Perdeuterated Lipid Acyl Chains of Membrane with Bound Viral Fusion Peptide Supports Large-Amplitude Motions of These Chains That Can Catalyze Membrane Fusion
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-26 , DOI: 10.1021/acs.biochem.1c00316 Ujjayini Ghosh 1 , David P Weliky 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-26 , DOI: 10.1021/acs.biochem.1c00316 Ujjayini Ghosh 1 , David P Weliky 1
Affiliation
|
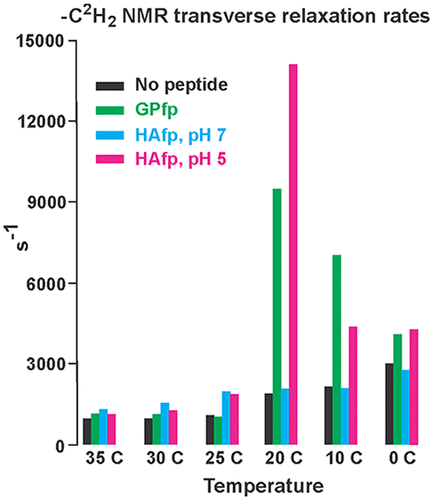
An early step in cellular infection by a membrane-enveloped virus like HIV or influenza is joining (fusion) of the viral and cell membranes. Fusion is catalyzed by a viral protein that typically includes an apolar “fusion peptide” (fp) segment that binds the target membrane prior to fusion. In this study, the effects of nonhomologous HIV and influenza fp’s on lipid acyl chain motion are probed with 2H NMR transverse relaxation rates (R2’s) of a perdeuterated DMPC membrane. Measurements were made between 35 and 0 °C, which brackets the membrane liquid-crystalline-to-gel phase transitions. Samples were made with either HIV “GPfp” at pH 7 or influenza “HAfp” at pH 5 or 7. GPfp induces vesicle fusion at pH 7, and HAfp induces more fusion at pH 5 vs 7. GPfp bound to DMPC adopts an intermolecular antiparallel β sheet structure, whereas HAfp is a monomer helical hairpin. The R2’s of the no peptide and HAfp, pH 7, samples increase gradually as temperature is lowered. The R2’s of GPfp and HAfp, pH 5, samples have very different temperature dependence, with a ∼10× increase in R2CD2 when temperature is reduced from 25 to 20 °C and smaller but still substantial R2’s at 10 and 0 °C. The large R2’s with GPfp and HAfp, pH 5, are consistent with large-amplitude motions of lipid acyl chains that can aid fusion catalysis by increasing the population of chains near the aqueous phase, which is the chain location for transition states between membrane fusion intermediates.
中文翻译:

具有结合病毒融合肽的膜的过氘化脂质酰基链的快速 2H NMR 横向弛豫支持可催化膜融合的这些链的大幅度运动
由膜包膜病毒(如 HIV 或流感)引起的细胞感染的早期步骤是病毒和细胞膜的结合(融合)。融合由病毒蛋白催化,该病毒蛋白通常包括在融合前结合靶膜的非极性“融合肽”(fp)片段。本研究采用2 H NMR 横向弛豫率 ( R 2's) 的过氘化 DMPC 膜。在 35 和 0 °C 之间进行测量,这包括了膜液晶到凝胶的相变。用 pH 7 的 HIV“GPfp”或 pH 5 或 7 的流感“HAfp”制备样品。GPfp 在 pH 7 诱导囊泡融合,HAfp 在 pH 5 对 7 诱导更多融合。与 DMPC 结合的 GPfp 采用分子间反平行β折叠结构,而HAfp是单体螺旋发夹。无肽和 HAfp(pH 7)样品的R 2值随着温度降低而逐渐增加。GPfp 和 HAfp的R 2 ,pH 5,样品具有非常不同的温度依赖性, R 2 CD2增加 ∼10 倍当温度从 25°C 降低到 20°C 和更小,但在 10°C 和 0°C 时R 2仍然很大。具有 GPfp 和 HAfp,pH 5的大R 2与脂质酰基链的大幅度运动一致,这可以通过增加水相附近的链数量来帮助融合催化,水相是过渡态之间的链位置膜融合中间体。
更新日期:2021-09-07
中文翻译:

具有结合病毒融合肽的膜的过氘化脂质酰基链的快速 2H NMR 横向弛豫支持可催化膜融合的这些链的大幅度运动
由膜包膜病毒(如 HIV 或流感)引起的细胞感染的早期步骤是病毒和细胞膜的结合(融合)。融合由病毒蛋白催化,该病毒蛋白通常包括在融合前结合靶膜的非极性“融合肽”(fp)片段。本研究采用2 H NMR 横向弛豫率 ( R 2's) 的过氘化 DMPC 膜。在 35 和 0 °C 之间进行测量,这包括了膜液晶到凝胶的相变。用 pH 7 的 HIV“GPfp”或 pH 5 或 7 的流感“HAfp”制备样品。GPfp 在 pH 7 诱导囊泡融合,HAfp 在 pH 5 对 7 诱导更多融合。与 DMPC 结合的 GPfp 采用分子间反平行β折叠结构,而HAfp是单体螺旋发夹。无肽和 HAfp(pH 7)样品的R 2值随着温度降低而逐渐增加。GPfp 和 HAfp的R 2 ,pH 5,样品具有非常不同的温度依赖性, R 2 CD2增加 ∼10 倍当温度从 25°C 降低到 20°C 和更小,但在 10°C 和 0°C 时R 2仍然很大。具有 GPfp 和 HAfp,pH 5的大R 2与脂质酰基链的大幅度运动一致,这可以通过增加水相附近的链数量来帮助融合催化,水相是过渡态之间的链位置膜融合中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号