The Journal of Supercritical Fluids ( IF 3.4 ) Pub Date : 2021-08-27 , DOI: 10.1016/j.supflu.2021.105387 Sarra Zid 1, 2 , Jean-Patrick Bazile 3 , Jean-Luc Daridon 3 , Andrés Piña-Martinez 4 , Jean-Noël Jaubert 4 , Stéphane Vitu 1, 2
|
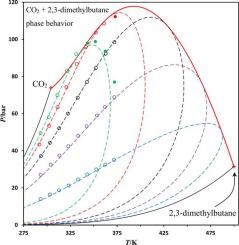
The phase behavior of the CO2 (1) + 2,3-dimethylbutane (2) binary mixture has been experimentally studied between 293 and 373 K. Saturation pressures, ranging from 14.0 to 112.2 bar, were visually measured using a high-pressure cell at carbon dioxide mole fractions between 0.20 and 0.93. 78 experimental points have been measured: 67 bubble points and 11 dew points. Experimental results reveal that the vapor-liquid critical locus is a continuous curve between the two pure compounds, meaning that the studied binary system exhibits a type I or II phase behavior in the classification scheme of Van Konynenburg and Scott. The experimental data can be very satisfactorily represented by the Peng-Robinson equation of state with mixing rules that embed a temperature-dependent binary interaction parameter.
中文翻译:

CO2 + 2,3-二甲基丁烷二元系统的液相平衡从 291.9 K 到 373.1 K
CO 2 (1) +的相行为 2,3-二甲基丁烷 (2) 二元混合物已在 293 至 373 K 之间进行了实验研究。饱和压力范围为 14.0 至 112.2 巴,使用高压池在 0.20 至 0.93 之间的二氧化碳摩尔分数下目测测量。已测量 78 个实验点:67 个泡点和 11 个露点。实验结果表明,汽液临界轨迹是两种纯化合物之间的连续曲线,这意味着所研究的二元体系在 Van Konynenburg 和 Scott 的分类方案中表现出 I 型或 II 型相行为。实验数据可以非常令人满意地由 Peng-Robinson 状态方程表示,其中混合规则嵌入了与温度相关的二元相互作用参数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号