Journal of Hepatology ( IF 25.7 ) Pub Date : 2021-08-27 , DOI: 10.1016/j.jhep.2021.07.037 Jens Ricke 1 , Regina Schinner 1 , Max Seidensticker 1 , Antonio Gasbarrini 2 , Otto M van Delden 3 , Holger Amthauer 4 , Bora Peynircioglu 5 , Irene Bargellini 6 , Roberto Iezzi 7 , Enrico N De Toni 8 , Peter Malfertheiner 9 , Maciej Pech 10 , Bruno Sangro 11
|
Background & Aims
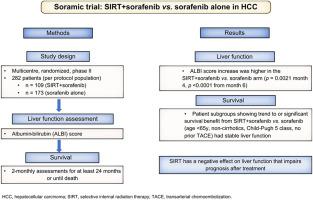
SORAMIC is a previously published randomised controlled trial assessing survival in patients with advanced hepatocellular carcinoma who received sorafenib with or without selective internal radiation therapy (SIRT). Based on the per-protocol (PP) population, we assessed whether the outcome of patients receiving SIRT+sorafenib vs. sorafenib alone was affected by adverse effects of SIRT on liver function.
Methods
The PP population consisted of 109 (SIRT+sorafenib) vs. 173 patients (sorafenib alone). Comparisons were made between subgroups who achieved a significant survival benefit or trend towards improved survival with SIRT and the inverse group without a survival benefit: <65 years-old vs. ≥65 years-old, Child-Pugh 5 vs. 6, no transarterial chemoembolisation (TACE) vs. prior TACE, no cirrhosis vs. cirrhosis, non-alcohol- vs. alcohol-related aetiology. The albumin-bilirubin (ALBI) score was used to monitor liver function over time during follow-up.
Results
ALBI scores increased in all patient groups during follow-up. In the PP population, ALBI score increases were higher in the SIRT+sorafenib than the sorafenib arm (p = 0.0021 month 4, p <0.0001 from month 6). SIRT+sorafenib conferred a survival benefit compared to sorafenib alone in patients aged <65 years-old, those without cirrhosis, those with Child-Pugh 5, and those who had not received TACE. A higher increase in ALBI score was observed in the inverse subgroups in whom survival was not improved by adding SIRT (age ≥65 years-old, p <0.05; cirrhosis, p = 0.07; Child-Pugh 6, p <0.05; prior TACE, p = 0.08).
Conclusion
SIRT frequently has a negative, often subclinical, effect on liver function in patients with hepatocellular carcinoma, which may impair prognosis after treatment. Careful patient selection for SIRT as well as prevention of clinical and subclinical liver damage by selective treatments, high tumour uptake ratio, and medical prophylaxis could translate into better efficacy.
Clinical trial number
EudraCT 2009-012576-27, NCT01126645
Lay summary
This study of treatments in patients with hepatocellular carcinoma found that selective internal radiation therapy (SIRT) has an adverse effect on liver function that may affect patient outcomes. Patients should be carefully selected before they undergo SIRT and the treatment technique should be optimised for maximum protection of non-target liver parenchyma.
中文翻译:

晚期肝细胞癌联合选择性内放射治疗或索拉非尼单药治疗后的肝功能☆
背景与目标
SORAMIC 是一项先前发表的随机对照试验,用于评估接受索拉非尼联合或不联合选择性内放射治疗 (SIRT) 的晚期肝细胞癌患者的生存率。基于符合方案 (PP) 人群,我们评估了接受 SIRT+索拉非尼与单独接受索拉非尼的患者的结果是否受到 SIRT 对肝功能的不利影响的影响。
方法
PP 人群由 109 名(SIRT+索拉非尼)和173 名患者(单独索拉非尼)组成。对通过 SIRT 获得显着生存获益或生存率改善趋势的亚组与没有生存获益的相反组进行比较:<65 岁对≥65 岁,Child-Pugh 5对6,无经动脉化疗栓塞 (TACE)与先前 TACE、无肝硬化与肝硬化、非酒精与酒精相关的病因。白蛋白-胆红素 (ALBI) 评分用于在随访期间随时间监测肝功能。
结果
随访期间所有患者组的 ALBI 评分均有所增加。在 PP 人群中,SIRT+索拉非尼组的 ALBI 评分增加高于索拉非尼组(第 4 个月p = 0.0021,第 6 个月p < 0.0001)。在年龄 <65 岁、无肝硬化、Child-Pugh 5 和未接受 TACE 的患者中,与单独使用索拉非尼相比,SIRT+索拉非尼可带来生存获益。在添加 SIRT 后生存率未改善的反向亚组中观察到 ALBI 评分增加较多(年龄≥65 岁,p < 0.05;肝硬化,p = 0.07;Child-Pugh 6,p < 0.05;既往 TACE , p = 0.08)。
结论
SIRT 经常对肝细胞癌患者的肝功能产生负面的(通常是亚临床的)影响,这可能会损害治疗后的预后。仔细选择 SIRT 的患者以及通过选择性治疗、高肿瘤摄取率和药物预防来预防临床和亚临床肝损伤可以转化为更好的疗效。
临床试验编号
EudraCT 2009-012576-27,NCT01126645
总结
这项针对肝细胞癌患者的治疗研究发现,选择性体内放射治疗 (SIRT) 对肝功能有不利影响,可能会影响患者的预后。在接受 SIRT 之前应仔细选择患者,并优化治疗技术以最大限度地保护非靶肝实质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号