Journal of Hepatology ( IF 26.8 ) Pub Date : 2021-08-27 , DOI: 10.1016/j.jhep.2021.08.018 Anne K Cordes 1 , Lilia Goudeva 2 , Marc Lütgehetmann 3 , Jürgen J Wenzel 4 , Patrick Behrendt 5 , Heiner Wedemeyer 5 , Albert Heim 1
|
Background and Aims
Immunocompromised patients are at risk of chronic hepatitis E which can be acquired by blood transfusions. Currently, screening of blood donors (BDs) for HEV RNA with a limit of detection (LOD) of 2,000 IU/ml is required in Germany. However, this may result in up to 440,000 IU of HEV RNA in blood products depending on their plasma volume. We studied the residual risk of transfusion-transmitted (tt) HEV infection when an LOD of 2,000 IU/ml is applied.
Methods
Highly sensitive individual donor testing for HEV RNA on the Grifols Procleix Panther system (LOD 7.89 IU/ml) was performed. HEV loads were quantified by real-time PCR.
Results
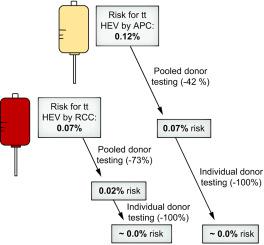
Of 16,236 donors, 31 (0.19%) were HEV RNA positive. Three BDs had viral loads between 710 and 2,000 IU/ml, which pose a significant risk of tt hepatitis E with any type of blood product. Eight BDs had viral loads of >32 to 710 IU/ml, which pose a risk of tt hepatitis E with platelet or plasma transfusions because of their higher plasma volume compared to red blood cell concentrates. Eight of these 11 potentially infectious BDs were seronegative for HEV, indicating a recent infection. Only 8 of 31 donors had viral loads >2,000 IU/ml that would also have been detected by the required screening procedure and 12 had very low HEV loads (<32 IU/ml).
Conclusions
Screening of BDs with an LOD of 2,000 IU/ml reduced the risk of tt HEV infection by about 73% for red blood cell concentrates but by just 42% for platelet and fresh frozen plasma transfusions. Single donor screening (LOD <32 IU/ml) should lead to an almost 100% risk reduction.
Lay summary
Immunocompromised patients, such as solid organ or hematopoietic stem cell recipients, are at risk of chronic hepatitis E, which can be acquired via blood transfusions. The risk of transfusion-transmitted hepatitis E in these patients may not be sufficiently controlled by (mini-)pool hepatitis E virus RNA screening of blood donors. Single donor screening should be considered to improve the safety of blood products.
中文翻译:

经混合测试的血小板和血浆导致输血传播戊型肝炎病毒的风险
背景和目标
免疫功能低下的患者有患慢性戊型肝炎的风险,可以通过输血获得。目前,德国需要对献血者 (BD) 进行 HEV RNA 筛查,检测限 (LOD) 为 2,000 IU/ml。然而,这可能会导致血液制品中的 HEV RNA 高达 440,000 IU,具体取决于其血浆量。我们研究了当 LOD 为 2,000 IU/ml 时输血传播 (tt) HEV 感染的残余风险。
方法
在 Grifols Procleix Panther 系统 (LOD 7.89 IU/ml) 上对 HEV RNA 进行了高度敏感的个体供体检测。HEV 负荷通过实时 PCR 进行量化。
结果
在 16,236 名捐赠者中,31 名 (0.19%) 为 HEV RNA 阳性。三个 BD 的病毒载量在 710 到 2,000 IU/ml 之间,这对使用任何类型的血液制品构成 tt 戊型肝炎的显着风险。8 个 BD 的病毒载量 > 32 至 710 IU/ml,由于与红细胞浓缩物相比,它们的血浆体积更高,因此存在血小板或血浆输注导致戊型肝炎的风险。这 11 种潜在传染性 BDs 中有 8 种对 HEV 呈血清阴性,表明最近有感染。31 名捐赠者中只有 8 名的病毒载量 >2,000 IU/ml,这也可以通过所需的筛查程序检测到,12 名的 HEV 载量非常低(<32 IU/ml)。
结论
筛查 LOD 为 2,000 IU/ml 的 BD 可使红细胞浓缩物感染 tt HEV 的风险降低约 73%,但血小板和新鲜冷冻血浆输注仅降低 42%。单供者筛查(LOD <32 IU/ml)应该可以降低几乎 100% 的风险。
总结
免疫功能低下的患者,例如实体器官或造血干细胞接受者,有患慢性戊型肝炎的风险,这可以通过输血获得。这些患者的输血传播戊型肝炎的风险可能无法通过献血者的(小型)汇集戊型肝炎病毒 RNA 筛查来充分控制。应考虑单供者筛查以提高血液制品的安全性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号