Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
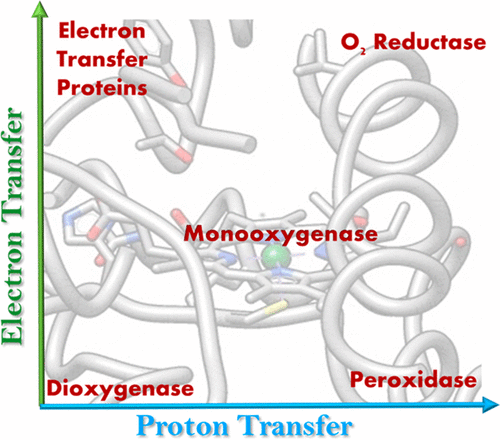
Rejigging Electron and Proton Transfer to Transition between Dioxygenase, Monooxygenase, Peroxygenase, and Oxygen Reduction Activity: Insights from Bioinspired Constructs of Heme Enzymes
JACS Au ( IF 8.5 ) Pub Date : 2021-08-26 , DOI: 10.1021/jacsau.1c00100 Manjistha Mukherjee 1 , Abhishek Dey 1
JACS Au ( IF 8.5 ) Pub Date : 2021-08-26 , DOI: 10.1021/jacsau.1c00100 Manjistha Mukherjee 1 , Abhishek Dey 1
Affiliation
|
Nature has employed heme proteins to execute a diverse set of vital life processes. Years of research have been devoted to understanding the factors which bias these heme enzymes, with all having a heme cofactor, toward distinct catalytic activity. Among them, axial ligation, distal super structure, and substrate binding pockets are few very vividly recognized ones. Detailed mechanistic investigation of these heme enzymes suggested that several of these enzymes, while functionally divergent, use similar intermediates. Furthermore, the formation and decay of these intermediates depend on proton and electron transfer processes in the enzyme active site. Over the past decade, work in this group, using in situ surface enhanced resonance Raman spectroscopy of synthetic and biosynthetic analogues of heme enzymes, a general idea of how proton and electron transfer rates relate to the lifetime of different O2 derived intermediates has been developed. These findings suggest that the enzymatic activities of all these heme enzymes can be integrated into one general cycle which can be branched out to different catalytic pathways by regulating the lifetime and population of each of these intermediates. This regulation can further be achieved by tuning the electron and proton transfer steps. By strategically populating one of these intermediates during oxygen reduction, one can navigate through different catalytic processes to a desired direction by altering proton and electron transfer steps.
中文翻译:

重新调整电子和质子转移以实现双加氧酶、单加氧酶、过氧酶和氧还原活性之间的转变:来自血红素酶的仿生结构的见解
大自然利用血红素蛋白来执行一系列不同的重要生命过程。多年来的研究致力于了解使这些血红素酶(所有酶都具有血红素辅因子)偏向于不同催化活性的因素。其中,轴向结扎、远端超级结构和底物结合口袋是很少见的。对这些血红素酶的详细机制研究表明,其中几种酶虽然功能不同,但使用相似的中间体。此外,这些中间体的形成和衰变取决于酶活性位点的质子和电子转移过程。在过去的十年中,该小组的工作使用血红素酶的合成和生物合成类似物的原位表面增强共振拉曼光谱,开发了质子和电子转移速率如何与不同 O 2衍生中间体的寿命相关的一般概念。这些发现表明,所有这些血红素酶的酶活性可以整合到一个通用循环中,通过调节每种中间体的寿命和数量,该循环可以分支到不同的催化途径。这种调节可以通过调整电子和质子转移步骤来进一步实现。通过在氧还原过程中策略性地填充这些中间体之一,人们可以通过改变质子和电子转移步骤来引导不同的催化过程到达所需的方向。
更新日期:2021-09-27
中文翻译:

重新调整电子和质子转移以实现双加氧酶、单加氧酶、过氧酶和氧还原活性之间的转变:来自血红素酶的仿生结构的见解
大自然利用血红素蛋白来执行一系列不同的重要生命过程。多年来的研究致力于了解使这些血红素酶(所有酶都具有血红素辅因子)偏向于不同催化活性的因素。其中,轴向结扎、远端超级结构和底物结合口袋是很少见的。对这些血红素酶的详细机制研究表明,其中几种酶虽然功能不同,但使用相似的中间体。此外,这些中间体的形成和衰变取决于酶活性位点的质子和电子转移过程。在过去的十年中,该小组的工作使用血红素酶的合成和生物合成类似物的原位表面增强共振拉曼光谱,开发了质子和电子转移速率如何与不同 O 2衍生中间体的寿命相关的一般概念。这些发现表明,所有这些血红素酶的酶活性可以整合到一个通用循环中,通过调节每种中间体的寿命和数量,该循环可以分支到不同的催化途径。这种调节可以通过调整电子和质子转移步骤来进一步实现。通过在氧还原过程中策略性地填充这些中间体之一,人们可以通过改变质子和电子转移步骤来引导不同的催化过程到达所需的方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号