当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aggregation and Significant Difference in Reactivity Therein: Blocking the CO2-to-CH3OH Reaction
Organometallics ( IF 2.5 ) Pub Date : 2021-08-27 , DOI: 10.1021/acs.organomet.1c00431 Xiaoyu Chen 1 , Dongli Wei 2 , Mårten S. G. Ahlquist 1
Organometallics ( IF 2.5 ) Pub Date : 2021-08-27 , DOI: 10.1021/acs.organomet.1c00431 Xiaoyu Chen 1 , Dongli Wei 2 , Mårten S. G. Ahlquist 1
Affiliation
|
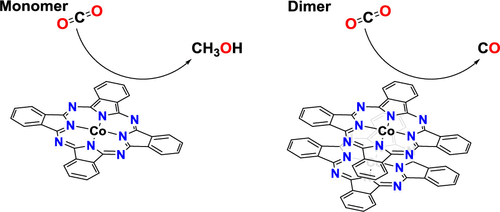
A CoPc/CNT system has been only recently reported to transform CO2 to methanol via electrochemical reductions, despite the fact that catalyst has been studied extensively since the 1980s. The explanation of high methanol selectivity lies behind the fact that in the new report CoPc exists mainly as a monomer, while in earlier works aggregates dominate. Here, we have studied the reactivity of monomeric and dimeric CoPc by DFT. The mechanism involves rate-limiting CO2 association, with the C–O cleavage step having very similar activation free energy. Once the Co–CO– intermediate is formed, the reaction bifurcates with two possible paths: (1) CO dissociation or (2) one additional reduction follows a protonation to give the Co–CHO– intermediate, which then leads to methanol by further reactions. For the monomeric species at low reduction potentials, CO dissociation is favored, but the formation of Co–CHO– becomes competitive at more negative applied potentials. For the dimer, the CO dissociation is always favored, and the reduction needed to form the C–H bond is negative enough for it not to be observed. The more difficult reduction stems from repulsive interactions between the CoPc units and lower solvent stabilization of the charge in the aggregate.
中文翻译:

聚集和其中反应性的显着差异:阻止 CO2-to-CH3OH 反应
CoPc/CNT 系统最近才被报道通过电化学还原将 CO 2转化为甲醇,尽管自 1980 年代以来已经对催化剂进行了广泛的研究。高甲醇选择性的解释在于,在新报告中 CoPc 主要作为单体存在,而在早期的工作中,聚集体占主导地位。在这里,我们通过 DFT 研究了单体和二聚体 CoPc 的反应性。该机制涉及限速 CO 2缔合,C-O 裂解步骤具有非常相似的活化自由能。一旦 Co-CO -中间体形成,反应就会通过两种可能的路径分叉:(1)CO 解离或(2)质子化后的额外还原得到 Co-CHO -中间体,然后通过进一步反应生成甲醇。在低还原电位的单体种类,CO解离是有利的,但共CHO的形成-变得有竞争力在更负的电位施加。对于二聚体,CO 解离总是有利的,并且形成 C-H 键所需的还原足够负以至于无法观察到。更困难的减少源于 CoPc 单元之间的排斥相互作用和聚集体中电荷的较低溶剂稳定性。
更新日期:2021-09-13
中文翻译:

聚集和其中反应性的显着差异:阻止 CO2-to-CH3OH 反应
CoPc/CNT 系统最近才被报道通过电化学还原将 CO 2转化为甲醇,尽管自 1980 年代以来已经对催化剂进行了广泛的研究。高甲醇选择性的解释在于,在新报告中 CoPc 主要作为单体存在,而在早期的工作中,聚集体占主导地位。在这里,我们通过 DFT 研究了单体和二聚体 CoPc 的反应性。该机制涉及限速 CO 2缔合,C-O 裂解步骤具有非常相似的活化自由能。一旦 Co-CO -中间体形成,反应就会通过两种可能的路径分叉:(1)CO 解离或(2)质子化后的额外还原得到 Co-CHO -中间体,然后通过进一步反应生成甲醇。在低还原电位的单体种类,CO解离是有利的,但共CHO的形成-变得有竞争力在更负的电位施加。对于二聚体,CO 解离总是有利的,并且形成 C-H 键所需的还原足够负以至于无法观察到。更困难的减少源于 CoPc 单元之间的排斥相互作用和聚集体中电荷的较低溶剂稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号