Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2021-08-27 , DOI: 10.1016/j.abb.2021.109022 Stéphanie Andrade 1 , Joana Angélica Loureiro 1 , Maria do Carmo Pereira 1
|
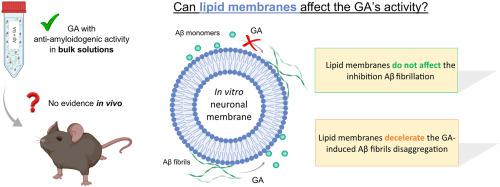
Molecules inhibiting the amyloid beta (Aβ) peptide aggregation and/or disaggregating mature fibrils are a promising approach for the Alzheimer's disease (AD) therapy, as the Aβ fibrillation is one of the key triggers of the disease. Gallic acid (GA) is a phenolic acid with anti-amyloidogenic activity against Aβ in buffered solutions. However, there is still no evidence of these properties in vivo. Given the rate of failures of AD drug development, there is a huge demand of replicating the in vivo environment in in vitro studies, thus allowing to stop earlier the study of molecules with no effect in vivo. Thus, this study aims to evaluate the effect of in vitro neuronal membranes on the GA's ability in preventing Aβ1-42 aggregation and disrupting preformed fibrils. To this end, liposomes were employed to mimic the cell membrane environment. The results reveal that the lipid membranes did not affect the GA's ability in inhibiting Aβ1−42 fibrillation. However, in vitro neuronal membranes modulate the GA-induced Aβ fibrils disaggregation, which may be related with the moderate affinity of the compound for the lipid membrane. Even so, GA presented strong anti-amyloidogenic properties in the cell membrane-like environment. This work highlights the promising value of GA on preventing and treating AD, thus justifying its study in animal models.
中文翻译:

体外神经元膜对没食子酸抗淀粉样蛋白活性的影响:对阿尔茨海默病治疗的意义
抑制淀粉样蛋白 β (Aβ) 肽聚集和/或分解成熟原纤维的分子是治疗阿尔茨海默病 (AD) 的一种很有前景的方法,因为 Aβ 纤维化是该疾病的关键触发因素之一。没食子酸 (GA) 是一种酚酸,在缓冲溶液中对 Aβ具有抗淀粉样蛋白生成活性。然而,在体内仍然没有这些特性的证据。鉴于 AD 药物开发的失败率,在体外研究中复制体内环境的需求很大,从而可以提前停止对体内没有影响的分子的研究。因此,本研究旨在评估体外神经元膜对 GA 阻止 Aβ 1-42聚集和破坏预先形成的原纤维的能力。为此,脂质体被用来模拟细胞膜环境。结果表明,脂质膜不影响 GA 抑制 Aβ 1-42纤维化的能力。然而,体外神经元膜调节 GA 诱导的 Aβ 原纤维解聚,这可能与化合物对脂质膜的中等亲和力有关。即便如此,GA在细胞膜样环境中表现出强大的抗淀粉样蛋白生成特性。这项工作突出了 GA 在预防和治疗 AD 方面的潜在价值,从而证明其在动物模型中的研究是合理的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号