Molecular Cell ( IF 14.5 ) Pub Date : 2021-08-27 , DOI: 10.1016/j.molcel.2021.07.040 Fansen Meng 1 , Zhengyang Yu 2 , Dan Zhang 3 , Shasha Chen 4 , Hongxin Guan 5 , Ruyuan Zhou 6 , Qirou Wu 2 , Qian Zhang 6 , Shengduo Liu 3 , Mukesh Kumar Venkat Ramani 7 , Bing Yang 2 , Xiao-Qun Ba 8 , Jing Zhang 8 , Jun Huang 2 , Xueli Bai 9 , Jun Qin 10 , Xin-Hua Feng 11 , Songying Ouyang 5 , Yan Jessie Zhang 7 , Tingbo Liang 12 , Pinglong Xu 13
|
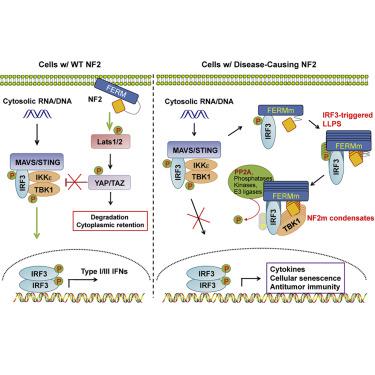
Missense mutations of the tumor suppressor Neurofibromin 2 (NF2/Merlin/schwannomin) result in sporadic to frequent occurrences of tumorigenesis in multiple organs. However, the underlying pathogenicity of NF2-related tumorigenesis remains mostly unknown. Here we found that NF2 facilitated innate immunity by regulating YAP/TAZ-mediated TBK1 inhibition. Unexpectedly, patient-derived individual mutations in the FERM domain of NF2 (NF2m) converted NF2 into a potent suppressor of cGAS-STING signaling. Mechanistically, NF2m gained extreme associations with IRF3 and TBK1 and, upon innate nucleic acid sensing, was directly induced by the activated IRF3 to form cellular condensates, which contained the PP2A complex, to eliminate TBK1 activation. Accordingly, NF2m robustly suppressed STING-initiated antitumor immunity in cancer cell-autonomous and -nonautonomous murine models, and NF2m-IRF3 condensates were evident in human vestibular schwannomas. Our study reports phase separation-mediated quiescence of cGAS-STING signaling by a mutant tumor suppressor and reveals gain-of-function pathogenesis for NF2-related tumors by regulating antitumor immunity.
中文翻译:

突变体 NF2 的诱导相分离禁锢了 cGAS-STING 机制,从而消除了抗肿瘤免疫
肿瘤抑制因子神经纤维蛋白 2 (NF2/Merlin/Schwannomin) 的错义突变导致多个器官中偶发或频繁发生肿瘤发生。然而,NF2 相关肿瘤发生的潜在致病性仍然大多未知。在这里,我们发现 NF2 通过调节 YAP/TAZ 介导的 TBK1 抑制来促进先天免疫。出乎意料的是,患者衍生的 NF2 FERM 结构域 (NF2m) 的个体突变将 NF2 转化为 cGAS-STING 信号传导的有效抑制剂。从机制上讲,NF2m 与 IRF3 和 TBK1 获得了极端的关联,并且在先天核酸感应后,被激活的 IRF3 直接诱导形成细胞凝聚物,其中含有 PP2A 复合物,以消除 TBK1 的激活。因此,NF2m 在癌细胞自主和非自主小鼠模型中强烈抑制 STING 引发的抗肿瘤免疫,并且 NF2m-IRF3 凝聚物在人前庭神经鞘瘤中很明显。我们的研究报告了突变型肿瘤抑制因子介导的相分离介导的 cGAS-STING 信号静止,并通过调节抗肿瘤免疫揭示了 NF2 相关肿瘤的功能获得性发病机制。










































 京公网安备 11010802027423号
京公网安备 11010802027423号