Molecular Catalysis ( IF 3.9 ) Pub Date : 2021-08-26 , DOI: 10.1016/j.mcat.2021.111785 Zheyuan Xu 1 , Deguang Liu 1 , Haizhu Yu 2 , Mårten S.G. Ahlquist 3 , Yao Fu 1
|
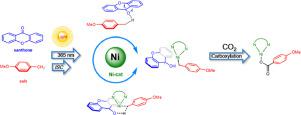
The photo carboxylation of the benzylic C(sp3)-H bond catalyzed by xanthone/nickel were examined by density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations. This study corroborates the previous proposal that light promotes the H-transfer from benzylic C(sp3)-H bond of the p-methoxytoluene to excited state of photocatalyst xanthone. Meanwhile, Ni(0) catalyst could mediate the H-transfer to occur via an electron-coupled-proton transfer manner, and then remarkably facilitates the carboxylation step (compared to the Ni-absent systems). After that, the generated Ni(I) intermediate and ketyl radical anion complete the carboxylation and electron transfer processes independently.
中文翻译:

氧杂蒽酮和Ni(0)催化剂光羧化苄基CH键的机理研究
通过密度泛函理论 (DFT) 和时间相关 DFT (TD-DFT) 计算检查了由氧杂蒽酮/镍催化的苄基 C( sp 3 )-H 键的光羧化。该研究证实了先前的提议,即光促进 H-从对甲氧基甲苯的苄基 C( sp 3 )-H 键转移到光催化剂氧杂蒽酮的激发态。同时,Ni(0) 催化剂可以通过电子耦合质子转移方式介导 H 转移发生,然后显着促进羧化步骤(与不含 Ni 的系统相比)。之后,生成的 Ni(I) 中间体和羰基自由基阴离子独立完成羧化和电子转移过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号