当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-directing effect of aqueous alkali metal ion (Li+, Na+ and K+) clusters on the polyoxoanion configuration in vanadium–molybdenum polyoxometalate solid solutions
CrystEngComm ( IF 2.6 ) Pub Date : 2021-08-05 , DOI: 10.1039/d1ce00875g Zhi-Yuan Yao 1, 2 , Guo-Qin Zhang 1 , Zi-Han Li 1 , Lin-Jiang Shen 2 , Xiao-Ming Ren 1, 3
CrystEngComm ( IF 2.6 ) Pub Date : 2021-08-05 , DOI: 10.1039/d1ce00875g Zhi-Yuan Yao 1, 2 , Guo-Qin Zhang 1 , Zi-Han Li 1 , Lin-Jiang Shen 2 , Xiao-Ming Ren 1, 3
Affiliation
|
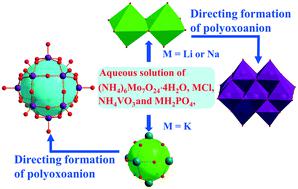
In a structural chemistry context, the dense packing and the structure-directing agent (SDA) often play a crucial role in the formation of the desired crystal structure. Herein, we present two isomorphous decavanadate-type polyoxometalates, M2(NH4)4−x[V10−xMoxO28]·10H2O (M = Li and x = 0.13 for 1; M = Na and x = 0.95 for 2), prepared by solution evaporation under ambient conditions and characterized by the techniques of EDS, ICP-MS, TG, XPS, X-band EPR spectroscopy, and X-ray single crystal and powder diffractions. Both 1 and 2 crystallized in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with analogous cell parameters, containing thermodynamically stable [M2(H2O)10]2+ (M = Li or Na) clusters, NH4+ ions and decavanadate-type polyoxoanions with V5+ and Mo6+ ions. The polyoxoanions and {M2(H2O)10}2+ cluster cations, owing to their comparable dimensions, arrange alternately to form dense packing along the a + c, c- and a-axis directions. As earlier reported, however, [(H2O)0.3@K6(H2O)12]H4.45[PV7.45Mo4.55O40]·11H2O (3) crystallizes in a CsCl-type dense packing structure under analogous crystal growth conditions. In 3, the thermodynamically-stable (H2O)0.3@K6(H2O)12 cluster cation and Keggin-type {Mo4.55V7.45PO40}10.45− polyoxoanion have comparable dimensions. This study clearly reveals the structure-directing effect of thermodynamically-stable aqueous alkali metal ion clusters, initially formed in the reaction mixture, on a polyoxoanion configuration in vanadium–molybdenum polyoxometalate solid solutions.
with analogous cell parameters, containing thermodynamically stable [M2(H2O)10]2+ (M = Li or Na) clusters, NH4+ ions and decavanadate-type polyoxoanions with V5+ and Mo6+ ions. The polyoxoanions and {M2(H2O)10}2+ cluster cations, owing to their comparable dimensions, arrange alternately to form dense packing along the a + c, c- and a-axis directions. As earlier reported, however, [(H2O)0.3@K6(H2O)12]H4.45[PV7.45Mo4.55O40]·11H2O (3) crystallizes in a CsCl-type dense packing structure under analogous crystal growth conditions. In 3, the thermodynamically-stable (H2O)0.3@K6(H2O)12 cluster cation and Keggin-type {Mo4.55V7.45PO40}10.45− polyoxoanion have comparable dimensions. This study clearly reveals the structure-directing effect of thermodynamically-stable aqueous alkali metal ion clusters, initially formed in the reaction mixture, on a polyoxoanion configuration in vanadium–molybdenum polyoxometalate solid solutions.
中文翻译:

含水碱金属离子(Li+、Na+和K+)簇对钒钼多金属氧酸盐固溶体中多氧阴离子构型的结构导向作用
在结构化学背景下,密集堆积和结构导向剂 (SDA) 通常在所需晶体结构的形成中起着至关重要的作用。在此,我们提出了两种同晶十钒酸盐型多金属氧酸盐,M 2 (NH 4 ) 4− x [V 10− x Mo x O 28 ]·10H 2 O(对于1,M = Li 和x = 0.13 ;M = Na 和x = 0.95 for 2 ),在环境条件下通过溶液蒸发制备,并通过 EDS、ICP-MS、TG、XPS、X 波段 EPR 光谱和 X 射线单晶和粉末衍射技术表征。两个都1和2在具有类似胞参数的空间群P 中结晶![[1 与组合宏]](https://www.rsc.org/images/entities/char_0031_0304.gif) ,包含热力学稳定的 [M 2 (H 2 O) 10 ] 2+ (M = Li 或 Na) 簇、NH 4 +离子和具有 V 5 的十钒酸盐型多氧阴离子+和 Mo 6+离子。多氧阴离子和 {M 2 (H 2 O) 10 } 2+簇阳离子,由于它们的尺寸相当,交替排列以沿a + c、c - 和a- 轴方向。然而,如前所述,[(H 2 O) 0.3 @K 6 (H 2 O) 12 ]H 4.45 [PV 7.45 Mo 4.55 O 40 ]·11H 2 O ( 3 ) 在 CsCl 型致密堆积结构中结晶类似的晶体生长条件。在3 中,热力学稳定的 (H 2 O) 0.3 @K 6 (H 2 O) 12簇阳离子和 Keggin 型 {Mo 4.55 V 7.45 PO 40 } 10.45−聚氧阴离子具有相当的尺寸。该研究清楚地揭示了最初在反应混合物中形成的热力学稳定的含水碱金属离子簇对钒-钼多金属氧酸盐固溶体中的多氧阴离子构型的结构导向作用。
,包含热力学稳定的 [M 2 (H 2 O) 10 ] 2+ (M = Li 或 Na) 簇、NH 4 +离子和具有 V 5 的十钒酸盐型多氧阴离子+和 Mo 6+离子。多氧阴离子和 {M 2 (H 2 O) 10 } 2+簇阳离子,由于它们的尺寸相当,交替排列以沿a + c、c - 和a- 轴方向。然而,如前所述,[(H 2 O) 0.3 @K 6 (H 2 O) 12 ]H 4.45 [PV 7.45 Mo 4.55 O 40 ]·11H 2 O ( 3 ) 在 CsCl 型致密堆积结构中结晶类似的晶体生长条件。在3 中,热力学稳定的 (H 2 O) 0.3 @K 6 (H 2 O) 12簇阳离子和 Keggin 型 {Mo 4.55 V 7.45 PO 40 } 10.45−聚氧阴离子具有相当的尺寸。该研究清楚地揭示了最初在反应混合物中形成的热力学稳定的含水碱金属离子簇对钒-钼多金属氧酸盐固溶体中的多氧阴离子构型的结构导向作用。
更新日期:2021-08-27
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with analogous cell parameters, containing thermodynamically stable [M2(H2O)10]2+ (M = Li or Na) clusters, NH4+ ions and decavanadate-type polyoxoanions with V5+ and Mo6+ ions. The polyoxoanions and {M2(H2O)10}2+ cluster cations, owing to their comparable dimensions, arrange alternately to form dense packing along the a + c, c- and a-axis directions. As earlier reported, however, [(H2O)0.3@K6(H2O)12]H4.45[PV7.45Mo4.55O40]·11H2O (3) crystallizes in a CsCl-type dense packing structure under analogous crystal growth conditions. In 3, the thermodynamically-stable (H2O)0.3@K6(H2O)12 cluster cation and Keggin-type {Mo4.55V7.45PO40}10.45− polyoxoanion have comparable dimensions. This study clearly reveals the structure-directing effect of thermodynamically-stable aqueous alkali metal ion clusters, initially formed in the reaction mixture, on a polyoxoanion configuration in vanadium–molybdenum polyoxometalate solid solutions.
with analogous cell parameters, containing thermodynamically stable [M2(H2O)10]2+ (M = Li or Na) clusters, NH4+ ions and decavanadate-type polyoxoanions with V5+ and Mo6+ ions. The polyoxoanions and {M2(H2O)10}2+ cluster cations, owing to their comparable dimensions, arrange alternately to form dense packing along the a + c, c- and a-axis directions. As earlier reported, however, [(H2O)0.3@K6(H2O)12]H4.45[PV7.45Mo4.55O40]·11H2O (3) crystallizes in a CsCl-type dense packing structure under analogous crystal growth conditions. In 3, the thermodynamically-stable (H2O)0.3@K6(H2O)12 cluster cation and Keggin-type {Mo4.55V7.45PO40}10.45− polyoxoanion have comparable dimensions. This study clearly reveals the structure-directing effect of thermodynamically-stable aqueous alkali metal ion clusters, initially formed in the reaction mixture, on a polyoxoanion configuration in vanadium–molybdenum polyoxometalate solid solutions.
中文翻译:

含水碱金属离子(Li+、Na+和K+)簇对钒钼多金属氧酸盐固溶体中多氧阴离子构型的结构导向作用
在结构化学背景下,密集堆积和结构导向剂 (SDA) 通常在所需晶体结构的形成中起着至关重要的作用。在此,我们提出了两种同晶十钒酸盐型多金属氧酸盐,M 2 (NH 4 ) 4− x [V 10− x Mo x O 28 ]·10H 2 O(对于1,M = Li 和x = 0.13 ;M = Na 和x = 0.95 for 2 ),在环境条件下通过溶液蒸发制备,并通过 EDS、ICP-MS、TG、XPS、X 波段 EPR 光谱和 X 射线单晶和粉末衍射技术表征。两个都1和2在具有类似胞参数的空间群P 中结晶
![[1 与组合宏]](https://www.rsc.org/images/entities/char_0031_0304.gif) ,包含热力学稳定的 [M 2 (H 2 O) 10 ] 2+ (M = Li 或 Na) 簇、NH 4 +离子和具有 V 5 的十钒酸盐型多氧阴离子+和 Mo 6+离子。多氧阴离子和 {M 2 (H 2 O) 10 } 2+簇阳离子,由于它们的尺寸相当,交替排列以沿a + c、c - 和a- 轴方向。然而,如前所述,[(H 2 O) 0.3 @K 6 (H 2 O) 12 ]H 4.45 [PV 7.45 Mo 4.55 O 40 ]·11H 2 O ( 3 ) 在 CsCl 型致密堆积结构中结晶类似的晶体生长条件。在3 中,热力学稳定的 (H 2 O) 0.3 @K 6 (H 2 O) 12簇阳离子和 Keggin 型 {Mo 4.55 V 7.45 PO 40 } 10.45−聚氧阴离子具有相当的尺寸。该研究清楚地揭示了最初在反应混合物中形成的热力学稳定的含水碱金属离子簇对钒-钼多金属氧酸盐固溶体中的多氧阴离子构型的结构导向作用。
,包含热力学稳定的 [M 2 (H 2 O) 10 ] 2+ (M = Li 或 Na) 簇、NH 4 +离子和具有 V 5 的十钒酸盐型多氧阴离子+和 Mo 6+离子。多氧阴离子和 {M 2 (H 2 O) 10 } 2+簇阳离子,由于它们的尺寸相当,交替排列以沿a + c、c - 和a- 轴方向。然而,如前所述,[(H 2 O) 0.3 @K 6 (H 2 O) 12 ]H 4.45 [PV 7.45 Mo 4.55 O 40 ]·11H 2 O ( 3 ) 在 CsCl 型致密堆积结构中结晶类似的晶体生长条件。在3 中,热力学稳定的 (H 2 O) 0.3 @K 6 (H 2 O) 12簇阳离子和 Keggin 型 {Mo 4.55 V 7.45 PO 40 } 10.45−聚氧阴离子具有相当的尺寸。该研究清楚地揭示了最初在反应混合物中形成的热力学稳定的含水碱金属离子簇对钒-钼多金属氧酸盐固溶体中的多氧阴离子构型的结构导向作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号