当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction of hydroxyl radical with arenes in solution—On the importance of benzylic hydrogen abstraction
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-08-26 , DOI: 10.1002/poc.4278 Abygail R. Waggoner 1 , Yahya Abdulrahman 2 , Alexis J. Iverson 2 , Ethan P. Gibson 1 , Mark A. Buckles 1 , James S. Poole 1, 2
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-08-26 , DOI: 10.1002/poc.4278 Abygail R. Waggoner 1 , Yahya Abdulrahman 2 , Alexis J. Iverson 2 , Ethan P. Gibson 1 , Mark A. Buckles 1 , James S. Poole 1, 2
Affiliation
|
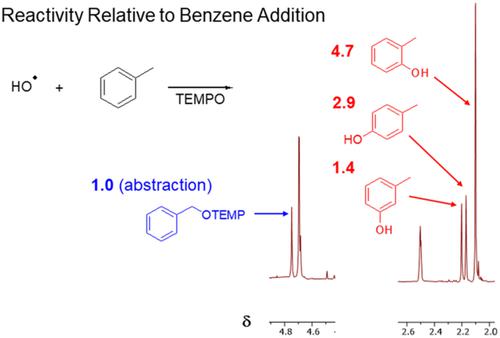
The regioselectivity of hydroxyl radical reactions with alkylarenes was investigated using a nuclear magnetic resonance (NMR)-based methodology capable of trapping and quantifying addition and hydrogen abstraction products of the initial elementary step of the oxidation process. Abstraction products are relatively minor components of the product mixtures (15–30 mol%), depending on the magnitude of the overall rate coefficient and the number of available hydrogens. The relative reactivity of addition at a given position on the ring depends on its relation to the methyl substituents on the hydrocarbons under study. The reactivity enhancements for disubstituted and trisubstituted rings are approximately additive under the conditions of this study.
中文翻译:

溶液中羟基与芳烃的反应——论苄基夺氢的重要性
使用基于核磁共振 (NMR) 的方法研究羟基自由基与烷基芳烃反应的区域选择性,该方法能够捕获和量化氧化过程初始基本步骤的加成和夺氢产物。提取产物是产物混合物中相对较少的组分 (15-30 mol%),具体取决于总速率系数的大小和可用氢的数量。在环上给定位置的加成的相对反应性取决于它与所研究烃上甲基取代基的关系。在本研究的条件下,二取代和三取代环的反应性增强大约是相加的。
更新日期:2021-08-26
中文翻译:

溶液中羟基与芳烃的反应——论苄基夺氢的重要性
使用基于核磁共振 (NMR) 的方法研究羟基自由基与烷基芳烃反应的区域选择性,该方法能够捕获和量化氧化过程初始基本步骤的加成和夺氢产物。提取产物是产物混合物中相对较少的组分 (15-30 mol%),具体取决于总速率系数的大小和可用氢的数量。在环上给定位置的加成的相对反应性取决于它与所研究烃上甲基取代基的关系。在本研究的条件下,二取代和三取代环的反应性增强大约是相加的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号