当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical study of enantioenriched aminohydroxylation of styrene catalyzed by an engineered hemoprotein
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-08-26 , DOI: 10.1002/poc.4280 Hong Huang 1 , Dong‐Xia Zhao 1 , Zhong‐Zhi Yang 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-08-26 , DOI: 10.1002/poc.4280 Hong Huang 1 , Dong‐Xia Zhao 1 , Zhong‐Zhi Yang 1
Affiliation
|
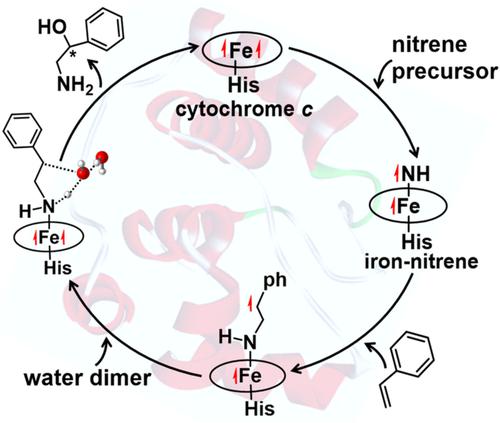
Transforming olefins to chiral amino alcohols is a useful approach to synthesize biologically active natural products and numerous drugs. A recent study has demonstrated a promising and synthetic value of an engineered hemoprotein for catalyzing olefins to chiral amino alcohols with 2500 total turnover numbers and 90% ee. Density functional theory (DFT) calculation has been used to systematically investigate the detailed mechanisms of the aforementioned process. One electron transfers from Fe atom to HN–nitrene in the iron–nitrene intermediate formation. Subsequently, styrene aziridination, singlet state is characterized by a nonradical, concerted nonsynchronous mechanism, while a radical and stepwise mechanism for triplet. Through hydrolysis reaction forming amino alcohol enantiomers, radical intermediate in triplet state without ring-opening process is obviously more feasible than singlet aziridine, where the energy barrier difference between triplet and singlet approaches to 20.00 kcal/mol. Moreover, due to the hydrogen bond effect, the water dimer-assisted hydrolysis reaction is effective to reduce the energy barrier by about 7.00 kcal/mol compared with one water assisted in triplet; however, the energy barrier difference in singlet is unapparent with only 0.18 kcal/mol accompanied with ring-opening process. Further, homology modeling shows that the reactivity and enantioselectivity can be attributed to the structure of the enzyme active pocket. This study sheds light on the mechanism of engineered hemoprotein-mediated amino alcohols synthesis and shows the development of biological catalysts.
中文翻译:

工程血红蛋白催化苯乙烯对映体富集氨基羟基化的理论研究
将烯烃转化为手性氨基醇是合成具有生物活性的天然产物和众多药物的有用方法。最近的一项研究证明了一种工程血红素蛋白在将烯烃催化为手性氨基醇的过程中具有广阔的合成价值,总转化率为 2500,ee 为 90%。密度泛函理论 (DFT) 计算已被用于系统地研究上述过程的详细机制。在铁-氮中间体形成中,一个电子从 Fe 原子转移到 HN-氮。随后,苯乙烯氮丙啶化,单线态的特点是非自由基,协调的非同步机制,而三线态的自由基和逐步机制。通过水解反应形成氨基醇对映体,没有开环过程的三线态自由基中间体显然比单线态氮丙啶更可行,其中三线态和单线态之间的能垒差接近20.00 kcal/mol。此外,由于氢键效应,水二聚体辅助水解反应与三重体辅助水相比,能有效降低约 7.00 kcal/mol 的能量势垒;然而,单线态的能垒差异并不明显,只有 0.18 kcal/mol 伴随开环过程。此外,同源性建模表明反应性和对映选择性可归因于酶活性口袋的结构。这项研究阐明了工程血红素蛋白介导的氨基醇合成的机制,并展示了生物催化剂的发展。
更新日期:2021-08-26
中文翻译:

工程血红蛋白催化苯乙烯对映体富集氨基羟基化的理论研究
将烯烃转化为手性氨基醇是合成具有生物活性的天然产物和众多药物的有用方法。最近的一项研究证明了一种工程血红素蛋白在将烯烃催化为手性氨基醇的过程中具有广阔的合成价值,总转化率为 2500,ee 为 90%。密度泛函理论 (DFT) 计算已被用于系统地研究上述过程的详细机制。在铁-氮中间体形成中,一个电子从 Fe 原子转移到 HN-氮。随后,苯乙烯氮丙啶化,单线态的特点是非自由基,协调的非同步机制,而三线态的自由基和逐步机制。通过水解反应形成氨基醇对映体,没有开环过程的三线态自由基中间体显然比单线态氮丙啶更可行,其中三线态和单线态之间的能垒差接近20.00 kcal/mol。此外,由于氢键效应,水二聚体辅助水解反应与三重体辅助水相比,能有效降低约 7.00 kcal/mol 的能量势垒;然而,单线态的能垒差异并不明显,只有 0.18 kcal/mol 伴随开环过程。此外,同源性建模表明反应性和对映选择性可归因于酶活性口袋的结构。这项研究阐明了工程血红素蛋白介导的氨基醇合成的机制,并展示了生物催化剂的发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号