当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sodium fluoride-rich solid electrolyte interphase for sodium–metal and sodium–oxygen batteries
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2021-08-26 , DOI: 10.1002/bkcs.12381 Sujung Kim 1 , Younguk Jung 1 , Jiwon Park 1 , Misun Hong 1 , Hye Ryung Byon 1
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2021-08-26 , DOI: 10.1002/bkcs.12381 Sujung Kim 1 , Younguk Jung 1 , Jiwon Park 1 , Misun Hong 1 , Hye Ryung Byon 1
Affiliation
|
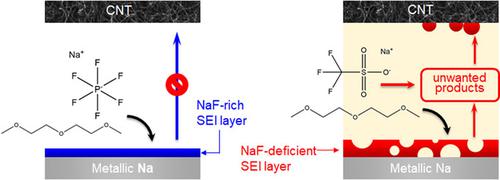
We studied solid electrolyte interphase (SEI) on metallic sodium (Na) electrodes. Among sodium hexafluorophosphate (NaPF6), sodium triflate (NaOTf), and sodium bis(trifluoromethanesulfonyl)imide (NaTFSI) electrolytes, sodium fluoride (NaF)-rich and compact SEI was only formed by chemical reduction of NaPF6. Excellent rigidity and insolubility of NaF-rich SEI layer enhanced electrochemical cycling performances for both Na/Na symmetric cells and sodium–oxygen (Na–O2) cells. By contrast, the Na electrodes using NaOTf and NaTFSI formed porous and carbonaceous SEI layers rather than NaF. Soluble carbonaceous species were detached from the Na electrode, which led to the undesired decomposition of electrolyte solution. It resulted in the substantial formation of the dead Na and dendritic Na and caused cycling failure of Na–O2 cells within 10 cycles, demonstrated by NaOTf.
中文翻译:

用于钠-金属和钠-氧电池的富含氟化钠的固体电解质界面
我们研究了金属钠 (Na) 电极上的固体电解质中间相 (SEI)。在六氟磷酸钠 (NaPF 6 )、三氟甲磺酸钠 (NaOTf) 和双(三氟甲磺酰) 亚胺 (NaTFSI) 电解质中,富含氟化钠 (NaF) 且致密的 SEI 仅通过 NaPF 6 的化学还原形成。富含 NaF 的 SEI 层优异的刚性和不溶性增强了 Na/Na 对称电池和钠-氧 (Na-O 2) 细胞。相比之下,使用 NaOTf 和 NaTFSI 的 Na 电极形成了多孔和碳质 SEI 层,而不是 NaF。可溶性碳质物质从钠电极上分离,导致电解质溶液发生不希望的分解。NaOTf 证明,它导致大量死亡 Na 和树突状 Na 的形成,并导致 Na-O 2细胞在 10 个循环内循环失败。
更新日期:2021-08-26
中文翻译:

用于钠-金属和钠-氧电池的富含氟化钠的固体电解质界面
我们研究了金属钠 (Na) 电极上的固体电解质中间相 (SEI)。在六氟磷酸钠 (NaPF 6 )、三氟甲磺酸钠 (NaOTf) 和双(三氟甲磺酰) 亚胺 (NaTFSI) 电解质中,富含氟化钠 (NaF) 且致密的 SEI 仅通过 NaPF 6 的化学还原形成。富含 NaF 的 SEI 层优异的刚性和不溶性增强了 Na/Na 对称电池和钠-氧 (Na-O 2) 细胞。相比之下,使用 NaOTf 和 NaTFSI 的 Na 电极形成了多孔和碳质 SEI 层,而不是 NaF。可溶性碳质物质从钠电极上分离,导致电解质溶液发生不希望的分解。NaOTf 证明,它导致大量死亡 Na 和树突状 Na 的形成,并导致 Na-O 2细胞在 10 个循环内循环失败。











































 京公网安备 11010802027423号
京公网安备 11010802027423号