Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2021-08-26 , DOI: 10.1016/j.colsurfb.2021.112068 Xiaoyu Wang 1 , Bin Gao 1 , Xiang-Kui Ren 1 , Jintang Guo 1 , Shihai Xia 2 , Wencheng Zhang 3 , Cheng Yang 4 , Yakai Feng 5
|
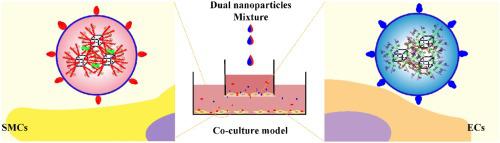
Inhibiting vascular restenosis remains a tricky challenge for the postoperative development of cardiovascular interventional therapy. The ideal approaches should activate endothelial cells (ECs) and restrain smooth muscle cells (SMCs), however, they are commonly contradictory. Herein, a strategy was developed for synchronizing ECs promotion and SMCs inhibition by codelivery DNA and siRNA for combination therapy. Thus, an easy and efficient strategy integrated dual-superiorities of precise targeting and dual therapeutic genes to precisely regulate the behaviors of ECs and SMCs. The ECs-targeting REDV peptide and SMCs-targeting VAPG peptide grafted anionic polymers were used to surface-functionalize the delivery nanoplatforms for vascular endothelial growth factor (VEGF) plasmids and ERK2 siRNA delivery, respectively. The dual targeting-nanoparticles were prepared by physical mixing method, and their outstanding advantages were confirmed by the co-culture experiments. In comparison with single targeting-nanoparticles and dual non-targeting-nanoparticles, the dual targeting-nanoparticles simultaneously enhanced ECs proliferation/migration and restrained SMCs proliferation/migration. Moreover, the dual targeting-nanoparticles group manifested the highest VEGF expression in ECs and the lowest ERK2 expression in SMCs. In summary, the two-pronged strategy with dual targeting-nanoparticles provides a valuable cornerstone for synchronizing ECs promotion and SMCs inhibition.
中文翻译:

通过双靶向纳米颗粒调节 ECs 和 SMCs 行为的两管齐下的方法
抑制血管再狭窄仍然是心血管介入治疗术后发展的一个棘手挑战。理想的方法应该激活内皮细胞 (ECs) 并抑制平滑肌细胞 (SMCs),然而,它们通常是相互矛盾的。在此,开发了一种策略,用于通过联合递送 DNA 和 siRNA 同步促进 ECs 和抑制 SMCs,以进行联合治疗。因此,一种简单有效的策略整合了精确靶向和双重治疗基因的双重优势,以精确调节 ECs 和 SMCs 的行为。分别使用靶向 ECs 的 REDV 肽和靶向 SMCs 的 VAPG 肽接枝阴离子聚合物对血管内皮生长因子 (VEGF) 质粒和 ERK2 siRNA 递送的递送纳米平台进行表面功能化。双靶向-纳米颗粒采用物理混合法制备,共培养实验证实了其突出优势。与单靶向纳米颗粒和双非靶向纳米颗粒相比,双靶向纳米颗粒同时增强了 ECs 增殖/迁移并抑制了 SMCs 增殖/迁移。此外,双靶向纳米颗粒组在 ECs 中表现出最高的 VEGF 表达和在 SMC 中最低的 ERK2 表达。总之,双靶向纳米颗粒的双管齐下的策略为同步 ECs 促进和 SMCs 抑制提供了宝贵的基石。与单靶向纳米颗粒和双非靶向纳米颗粒相比,双靶向纳米颗粒同时增强了 ECs 增殖/迁移并抑制了 SMCs 增殖/迁移。此外,双靶向纳米颗粒组在 ECs 中表现出最高的 VEGF 表达和在 SMC 中最低的 ERK2 表达。总之,双靶向纳米颗粒的双管齐下的策略为同步 ECs 促进和 SMCs 抑制提供了宝贵的基石。与单靶向纳米颗粒和双非靶向纳米颗粒相比,双靶向纳米颗粒同时增强了 ECs 增殖/迁移并抑制了 SMCs 增殖/迁移。此外,双靶向纳米颗粒组在 ECs 中表现出最高的 VEGF 表达和在 SMC 中最低的 ERK2 表达。总之,双靶向纳米颗粒的双管齐下的策略为同步 ECs 促进和 SMCs 抑制提供了宝贵的基石。











































 京公网安备 11010802027423号
京公网安备 11010802027423号