Anaerobe ( IF 2.3 ) Pub Date : 2021-08-25 , DOI: 10.1016/j.anaerobe.2021.102439 Sabina Noreen Wuersching 1 , Karin Christine Huth 1 , Reinhard Hickel 1 , Maximilian Kollmuss 1
|
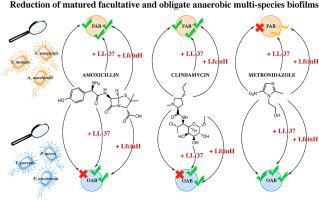
Antimicrobial peptides are receiving increasing attention as potential therapeutic agents for treating biofilm-related infections of the oral cavity. Many bacteria residing in biofilms exhibit an enhanced antibiotic tolerance, which grants intrinsically susceptible microorganisms to survive lethal concentrations of antibiotics. In this study, we examined the effects of two endogenous human antimicrobial peptides, LL-37 and human Lactoferricin, on the antibiotic drug efficacy of amoxicillin, clindamycin and metronidazole in two types of polymicrobial biofilms, which aimed to represent frequent oral diseases: (1) facultative anaerobic (Streptococcus mutans, Streptococcus sanguinis, Actinomyces naeslundii) and (2) obligate anaerobic biofilms (Veillonella parvula, Parvimonas micra, Fusobacterium nucleatum). LL-37 and Lactoferricin enhanced the anti-biofilm effect of amoxicillin and clindamycin in facultative anaerobic biofilms. Metronidazole alone was ineffective against facultative anaerobic biofilms, but the presence of LL-37 and Lactoferricin led to a greater biofilm reduction. Obligate anaerobic biofilms showed an increased drug tolerance to amoxicillin and clindamycin, presumably due to metabolic downshifts of the bacteria residing within the biofilm. However, when combined with LL-37 or Lactoferricin, the reduction of obligate anaerobic biofilms was markedly enhanced for all antibiotics, even for amoxicillin and clindamycin. Furthermore, our results suggest that antimicrobial peptides enhance the dispersion of matured biofilms, which may be one of their mechanisms for targeting biofilms. In summary, our study proves that antimicrobial peptides can serve as an auxiliary treatment strategy for combatting enhanced antibiotic tolerance in bacterial biofilms.
中文翻译:

靶向与口腔疾病相关的厌氧生物膜中的抗生素耐受性:人类抗菌肽 LL-37 和乳铁蛋白增强了阿莫西林、克林霉素和甲硝唑的抗生素疗效
抗菌肽作为治疗口腔生物膜相关感染的潜在治疗剂正受到越来越多的关注。许多存在于生物膜中的细菌表现出增强的抗生素耐受性,这使得本质上易感微生物能够在致命浓度的抗生素中存活。在这项研究中,我们检测了两种内源性人抗菌肽 LL-37 和人乳铁蛋白对阿莫西林、克林霉素和甲硝唑在两种类型的多微生物生物膜中的抗菌药物疗效的影响,旨在代表常见的口腔疾病:(1 )兼性厌氧菌(变形链球菌、血链球菌、内氏放线菌)和(2)专性厌氧生物膜(细小韦荣球菌,微单胞菌,具核梭杆菌)。LL-37 和乳铁蛋白增强了阿莫西林和克林霉素在兼性厌氧生物膜中的抗生物膜作用。单独使用甲硝唑对兼性厌氧生物膜无效,但 LL-37 和乳铁素的存在导致更大的生物膜减少。专性厌氧生物膜显示出对阿莫西林和克林霉素的药物耐受性增加,这可能是由于生物膜内细菌的代谢下降所致。然而,当与 LL-37 或乳铁霉素联合使用时,所有抗生素的专性厌氧生物膜的减少都显着增强,即使是阿莫西林和克林霉素也是如此。此外,我们的研究结果表明,抗菌肽增强了成熟生物膜的分散,这可能是它们靶向生物膜的机制之一。总之,



























 京公网安备 11010802027423号
京公网安备 11010802027423号