Process Biochemistry ( IF 4.4 ) Pub Date : 2021-08-25 , DOI: 10.1016/j.procbio.2021.08.022 Sadam Munawar 1 , Muhammad Sagir 2 , Ghulam Mustafa 1 , Muhammad Amjad Ali 3 , Adnan Khan Niazi 1 , Aqsa Parvaiz 1 , Farkhanda Yasmin 4 , Farukh Mansoor 5 , Shamsa Kanwal 5 , Majeeda Rasheed 6 , Hafiza Kehfulvara 1 , Habib Ali 7, 8 , Sami Ullah 9 , Abdullah G. Al-Sehemi 9 , Muhammad Sarwar Khan 1 , Faiz Ahmad Joyia 1
|
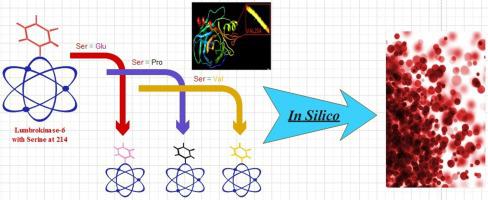
Lumbrokinases (LKs) belong to the group serine proteases capable to prevent thrombosis through the proteolysis of both plasminogen-bound and plasminogen-free fibrin molecules. The article presents improved activity of Lumbrokinase-6 (Lk-6) by suggesting the substitution of a Serine found at position 214 (Lk-6) with three other amino acids namely Glutamic acid, Proline and Valine. To characterize the stability, enzyme-substrate interaction and improved activity of three mutant Lk-6 proteins (Lk-Glu214, Lk-Pro214, Lk-Val214) In Silico tools were utilized. Subsequently, Lk-6 wild type and three mutant proteins were subjected to structure prediction, molecular modeling, phylogeny, molecular docking and Protein-Protein Interaction (PPI) using the In Silico tools. Collection and analysis of results revealed that substituted mutation at Ser214 with Valine214 can appreciably stabilize the overall structure of Lk-6 protein and makes its interaction with plasminogen activator physically powerful for higher plasmin activation. Similarly, Serine214 to Valine214 substitution resulted the direct activation of plasmin breakage at the Arg561-Val562 bond. The Arg-Val at position 561–562 in plasminogen and its connection at catalytic site have significantly shown that the predicted residue Valine214 could be further examined through genetic engineering of Lk-6 protein. Therefore, such results are potential steps towards the engineering of smart and active Lks.
中文翻译:

对纤溶蛋白“Lumbrokinase-6”中预测取代的计算机分析表明活性增强
Lumbrokinases (LKs) 属于丝氨酸蛋白酶组,能够通过结合纤溶酶原和不含纤溶酶原的纤维蛋白分子的蛋白水解来预防血栓形成。该文章通过建议用其他三个氨基酸,即谷氨酸、脯氨酸和缬氨酸替换在位置 214 (Lk-6) 处发现的丝氨酸,展示了 Lumbrokinase-6 (Lk-6) 的活性提高。为了表征三种突变 Lk-6 蛋白(Lk-Glu214、Lk-Pro214、Lk-Val214)的稳定性、酶-底物相互作用和改进的活性,使用了硅胶工具。随后,Lk-6 野生型和三种突变蛋白使用In Silico进行结构预测、分子建模、系统发育、分子对接和蛋白质-蛋白质相互作用 (PPI)。工具。结果的收集和分析表明,在 Ser214 处用缬氨酸 214 取代突变可以明显稳定 Lk-6 蛋白的整体结构,并使其与纤溶酶原激活剂的相互作用在物理上强大到更高的纤溶酶激活。类似地,丝氨酸 214 到缬氨酸 214 的取代导致 Arg561-Val562 键处纤溶酶断裂的直接激活。纤溶酶原第 561-562 位的 Arg-Val 及其在催化位点的连接已显着表明预测的残基缬氨酸 214 可以通过 Lk-6 蛋白的基因工程进一步检查。因此,这样的结果是实现智能和主动 Lks 工程的潜在步骤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号