当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Domino Ring-Opening of N-Tosyl Vinylaziridines Triggered by Aryne Diels-Alder Reaction
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-08-25 , DOI: 10.1002/adsc.202100697 Jiupeng Liu 1 , Jiaqi Li 1 , Bowen Ren 1 , Yun Zhang 1 , Linyi Xue 1 , Yanying Wang 1 , Jingjing Zhao 1 , Puyu Zhang 1 , Xuejun Xu 1 , Pan Li 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-08-25 , DOI: 10.1002/adsc.202100697 Jiupeng Liu 1 , Jiaqi Li 1 , Bowen Ren 1 , Yun Zhang 1 , Linyi Xue 1 , Yanying Wang 1 , Jingjing Zhao 1 , Puyu Zhang 1 , Xuejun Xu 1 , Pan Li 1
Affiliation
|
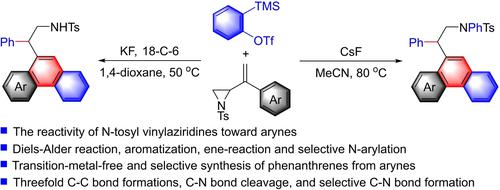
The reactivity of N-tosyl vinylaziridines toward arynes has been demonstrated under mild and transition-metal-free conditions to yield 2-(phenanthren-9-yl)ethan-1-sulfamides in moderate to good yields. The selective synthesis of N-H and N-aryl products was accomplished using CsF in MeCN and KF/18-C-6 in 1,4-dioxane, respectively. This cascade process involves a sequential Diels-Alder reaction, ring-opening aromatization, ene-reaction, and selective N-arylation reaction.
中文翻译:

由 Aryne Diels-Alder 反应引发的 N-甲苯磺酰基乙烯基氮丙啶的多米诺开环
N-甲苯磺酰基乙烯基氮丙啶对芳烃的反应性已在温和且无过渡金属的条件下得到证明,以中等至良好的产率生成 2-(phenanthren-9-yl)ethan-1-sulfamides。N- H 和N-芳基产物的选择性合成分别使用 MeCN 中的 CsF 和 1,4-二恶烷中的 KF/18-C-6 完成。该级联过程涉及连续的 Diels-Alder 反应、开环芳构化、烯反应和选择性N-芳基化反应。
更新日期:2021-10-19
中文翻译:

由 Aryne Diels-Alder 反应引发的 N-甲苯磺酰基乙烯基氮丙啶的多米诺开环
N-甲苯磺酰基乙烯基氮丙啶对芳烃的反应性已在温和且无过渡金属的条件下得到证明,以中等至良好的产率生成 2-(phenanthren-9-yl)ethan-1-sulfamides。N- H 和N-芳基产物的选择性合成分别使用 MeCN 中的 CsF 和 1,4-二恶烷中的 KF/18-C-6 完成。该级联过程涉及连续的 Diels-Alder 反应、开环芳构化、烯反应和选择性N-芳基化反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号