Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2021-08-26 , DOI: 10.1016/j.jmb.2021.167188 Biao Yuan 1 , Athina G Portaliou 2 , Rinky Parakra 2 , Jochem H Smit 3 , Jiri Wald 4 , Yichen Li 5 , Bindu Srinivasu 2 , Maria S Loos 2 , Harveer Singh Dhupar 6 , Dirk Fahrenkamp 4 , Charalampos G Kalodimos 7 , Franck Duong van Hoa 6 , Thorben Cordes 8 , Spyridoula Karamanou 2 , Thomas C Marlovits 4 , Anastassios Economou 2
|
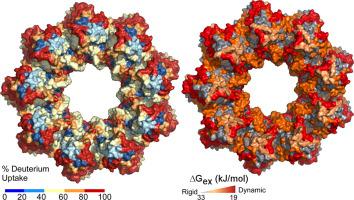
Type III protein secretion is widespread in Gram-negative pathogens. It comprises the injectisome with a surface-exposed needle and an inner membrane translocase. The translocase contains the SctRSTU export channel enveloped by the export gate subunit SctV that binds chaperone/exported clients and forms a putative ante-chamber. We probed the assembly, function, structure and dynamics of SctV from enteropathogenic E. coli (EPEC). In both EPEC and E. coli lab strains, SctV forms peripheral oligomeric clusters that are detergent-extracted as homo-nonamers. Membrane-embedded SctV9 is necessary and sufficient to act as a receptor for different chaperone/exported protein pairs with distinct C-domain binding sites that are essential for secretion. Negative staining electron microscopy revealed that peptidisc-reconstituted His-SctV9 forms a tripartite particle of ∼22 nm with a N-terminal domain connected by a short linker to a C-domain ring structure with a ∼5 nm-wide inner opening. The isolated C-domain ring was resolved with cryo-EM at 3.1 Å and structurally compared to other SctV homologues. Its four sub-domains undergo a three-stage “pinching” motion. Hydrogen-deuterium exchange mass spectrometry revealed this to involve dynamic and rigid hinges and a hyper-flexible sub-domain that flips out of the ring periphery and binds chaperones on and between adjacent protomers. These motions are coincident with local conformational changes at the pore surface and ring entry mouth that may also be modulated by the ATPase inner stalk. We propose that the intrinsic dynamics of the SctV protomer are modulated by chaperones and the ATPase and could affect allosterically the other subunits of the nonameric ring during secretion.
中文翻译:

功能性 Nonameric Type III Translocase Export Gate 的结构动力学
III 型蛋白分泌在革兰氏阴性病原体中很普遍。它包括带有暴露于表面的针头和内膜转位酶的注射体。转位酶包含 SctRSTU 出口通道,由出口门亚基 SctV 包裹,该通道结合伴侣/出口客户并形成假定的前室。我们探讨了来自肠致病性大肠杆菌(EPEC)的 SctV 的组装、功能、结构和动力学。在 EPEC 和大肠杆菌实验室菌株中,SctV 形成外围寡聚簇,这些簇被去污剂提取为同源异构体。膜嵌入 SctV 9作为具有不同 C 域结合位点的不同分子伴侣/输出蛋白对的受体是必要和足够的,这些位点对分泌至关重要。负染色电子显微镜显示肽盘重组的 His-SctV 9形成一个约 22 nm 的三方粒子,其 N 端域通过短接头连接到具有约 5 nm 宽内部开口的 C 域环结构。分离的 C 域环用 3.1 Å 的冷冻电镜解析,并在结构上与其他 SctV 同系物进行了比较。它的四个子域经历了三个阶段的“收缩”运动。氢-氘交换质谱表明这涉及动态和刚性铰链以及一个超柔性子域,该子域从环外围翻转并结合相邻原体上和之间的分子伴侣。这些运动与孔表面和环入口处的局部构象变化相一致,这也可能受 ATPase 内部茎的调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号