Journal of Hepatology ( IF 26.8 ) Pub Date : 2021-08-26 , DOI: 10.1016/j.jhep.2021.08.008 Paul J Thuluvath 1 , Polly Robarts 2 , Mahak Chauhan 2
|
Background & Aims
Liver transplant (LT) recipients or other immunocompromised patients were not included in the registration trials studying the efficacy of vaccines against SARS-CoV-2. Although the clinical efficacy of COVID-19 vaccines in immunocompromised patients is unknown, many societies have recommended vaccination of this highly vulnerable patient population.
Methods
In this prospective study, we determined antibody responses to spike protein, 4 weeks after the 2nd dose of mRNA vaccines or after the single dose of Johnson & Johnson vaccine, in LT recipients and those with chronic liver disease (CLD) with and without cirrhosis.
Results
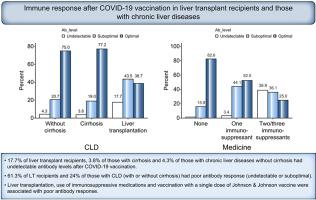
Of the 233 patients enrolled so far, 62 were LT recipients, 79 had cirrhosis (10 decompensated) and 92 had CLD without cirrhosis. Antibody titers were defined as undetectable (<0.40 U/ml), suboptimal (0.40–250 U/ml) and adequate (>250 U/ml). Of the 62 patients who had LT, antibody levels were undetectable in 11 patients and suboptimal (median titer 17.6, range 0.47–212 U/ml) in 27 patients. Among 79 patients with cirrhosis, 3 had undetectable antibody levels and 15 had suboptimal (median titer 41.3, range 0.49–221 U/L) antibody responses. Of the 92 patients without cirrhosis, 4 had undetectable antibody levels and 19 had suboptimal (median titer 95.5, range 4.9–234 U/L) antibody responses. Liver transplantation, use of 2 or more immunosuppression medications and vaccination with a single dose of the Johnson & Johnson vaccine were associated with poor immune response on multivariable analysis. No patient had any serious adverse events.
Conclusions
Poor antibody responses after SARS-CoV-2 vaccination were seen in 61% of LT recipients and 24% of those with CLD.
Lay summary
The clinical efficacy of COVID-19 vaccines in immunocompromised patients is unknown. We performed a prospective study to evaluate immune responses to COVID-19 vaccines (Moderna, Pfizer or Johnson & Johnson) in 62 liver transplant recipients, 79 patients with cirrhosis and 92 with chronic liver diseases without cirrhosis. We found that 17.8% of liver transplant recipients, 3.8% of those with cirrhosis and 4.3% of those with chronic liver diseases without cirrhosis had undetectable antibody levels. In total, 61.3% of liver transplant recipients and 24% of those with chronic liver diseases (with or without cirrhosis) had poor antibody responses (undetectable or suboptimal). Liver transplantation, use of immunosuppressive medications and vaccination with a single dose of Johnson & Johnson vaccine were associated with poor antibody responses when adjusted for other factors.
中文翻译:

肝移植受者和慢性肝病患者接种 COVID-19 后抗体反应分析
背景与目标
研究 SARS-CoV-2 疫苗功效的注册试验不包括肝移植 (LT) 接受者或其他免疫功能低下的患者。尽管 COVID-19 疫苗在免疫功能低下患者中的临床疗效尚不清楚,但许多社会已建议对这一高度脆弱的患者群体进行疫苗接种。
方法
在这项前瞻性研究中,我们确定了在 LT 受者和伴有和不伴有肝硬化的慢性肝病 (CLD) 患者中,在第 2 剂 mRNA 疫苗后 4 周或在单剂强生疫苗后4周,抗体对尖峰蛋白的反应.
结果
在迄今为止登记的 233 名患者中,62 名是 LT 接受者,79 名患有肝硬化(10 名失代偿),92 名患有非肝硬化的 CLD。抗体滴度定义为检测不到(<0.40 U/ml)、次优(0.40-250 U/ml)和充足(>250 U/ml)。在 62 名 LT 患者中,11 名患者的抗体水平检测不到,27 名患者的抗体水平不理想(中位滴度 17.6,范围 0.47-212 U/ml)。在 79 名肝硬化患者中,3 名抗体水平检测不到,15 名抗体反应不佳(中位滴度 41.3,范围 0.49-221 U/L)。在 92 名无肝硬化患者中,4 名抗体水平检测不到,19 名抗体反应欠佳(中位滴度 95.5,范围 4.9-234 U/L)。肝移植、使用 2 种或更多免疫抑制药物和单剂强生公司的疫苗接种 在多变量分析中,约翰逊疫苗与较差的免疫反应有关。没有患者发生任何严重的不良事件。
结论
在 61% 的 LT 接受者和 24% 的 CLD 接受者中观察到 SARS-CoV-2 疫苗接种后抗体反应不佳。
总结
COVID-19 疫苗在免疫功能低下患者中的临床疗效尚不清楚。我们进行了一项前瞻性研究,以评估 62 名肝移植受者、79 名肝硬化患者和 92 名无肝硬化慢性肝病患者对 COVID-19 疫苗(Moderna、辉瑞或强生)的免疫反应。我们发现,17.8% 的肝移植受者、3.8% 的肝硬化患者和 4.3% 的没有肝硬化的慢性肝病患者的抗体水平检测不到。总体而言,61.3% 的肝移植受者和 24% 的慢性肝病患者(有或没有肝硬化)的抗体反应较差(检测不到或次优)。肝移植、免疫抑制药物的使用和单剂量强生公司的疫苗接种











































 京公网安备 11010802027423号
京公网安备 11010802027423号