International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2021-08-26 , DOI: 10.1016/j.ijms.2021.116687 Gongyu Li 1, 2, 3 , Junfeng Huang 1 , Zhen Zheng 1 , Qinjingwen Cao 4 , Yuwei Tian 2 , Guangming Huang 3 , Lingjun Li 1, 4 , Brandon T. Ruotolo 2
|
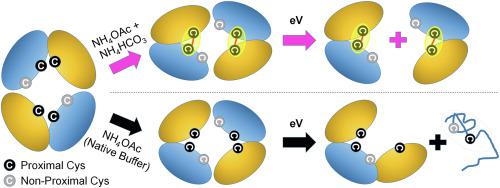
Previously, we have reported the stabilization effect of Hofmeister salts for multiprotein complexes (MPC) in the absence of bulk solvent (J. Am. Chem. Soc. 2011, 133 (29), 11,358–11367; Angew. Chem. Int. Ed. 2012, 51 (23), 5692–5695; Angew. Chem. Int. Ed. 2013, 52 (32), 8329–8332.). Our efforts sought to bridge the gap between gas-phase protein structures and those found in solution. To reveal more detailed MPC topology information, native ion mobility-mass spectrometry (IM-MS) measurements are often combined with gas-phase activation methods. Conventional activation methods, including collision induced dissociation/unfolding (CID/CIU), however, primarily report information focused on monomeric subunits within the MPC, limiting the topological information obtained. Herein, we describe a simple buffer-doping method that promotes an alternative MPC CID pathway which readily produces product ions that correspond to larger sub-complexes from within some parent assemblies. Interestingly, tetramers exhibiting a dimer of dimers quaternary structure (e.g. hemoglobin and concanavalin A) produce dimeric product ions upon collisional activation following ionization from bicarbonate buffer, in contrast to the commonly observed monomer-ejection CID pathway. In order to both further investigate and validate our native IM-MS, we performed bottom-up proteomics experiments on MPCs housed in bicarbonate buffer. Our efforts revealed evidence of bicarbonate-mediated disulfide bond formation in proximal Cystine residues. We close by discussing the applications for these observations in the context of MPC structure determination by native IM-MS.
中文翻译:

碳酸氢盐缓冲液可促进多蛋白复合物的交联和替代气相解离途径
此前,我们已经报道了霍夫迈斯特盐在没有大量溶剂的情况下对多蛋白复合物 (MPC) 的稳定作用 ( J. Am. Chem. Soc. 2011, 133 (29), 11,358–11367; Angew. Chem. Int. Ed . 2012, 51 (23), 5692–5695;Angew. Chem. Int. Ed. 2013 , 52(32), 8329–8332.)。我们的努力试图弥合气相蛋白质结构与溶液中发现的结构之间的差距。为了揭示更详细的 MPC 拓扑信息,天然离子迁移率 - 质谱 (IM-MS) 测量通常与气相活化方法相结合。然而,传统的激活方法,包括碰撞诱导解离/展开 (CID/CIU),主要报告集中在 MPC 内单体亚基的信息,限制了获得的拓扑信息。在本文中,我们描述了一种简单的缓冲掺杂方法,该方法促进了替代 MPC CID 途径,该途径很容易产生与某些母组件内较大的子复合物相对应的产物离子。有趣的是,四聚体表现出二聚体四级结构的二聚体(例如 血红蛋白和伴刀豆球蛋白 A) 在从碳酸氢盐缓冲液电离后碰撞激活时产生二聚产物离子,这与通常观察到的单体喷射 CID 途径相反。为了进一步研究和验证我们的原生 IM-MS,我们对放置在碳酸氢盐缓冲液中的 MPC 进行了自下而上的蛋白质组学实验。我们的努力揭示了在近端胱氨酸残基中碳酸氢盐介导的二硫键形成的证据。我们通过在本机 IM-MS 确定 MPC 结构的背景下讨论这些观察的应用来结束。我们对置于碳酸氢盐缓冲液中的 MPC 进行了自下而上的蛋白质组学实验。我们的努力揭示了在近端胱氨酸残基中碳酸氢盐介导的二硫键形成的证据。我们通过在本机 IM-MS 确定 MPC 结构的背景下讨论这些观察的应用来结束。我们对置于碳酸氢盐缓冲液中的 MPC 进行了自下而上的蛋白质组学实验。我们的努力揭示了在近端胱氨酸残基中碳酸氢盐介导的二硫键形成的证据。我们通过在本机 IM-MS 确定 MPC 结构的背景下讨论这些观察的应用来结束。











































 京公网安备 11010802027423号
京公网安备 11010802027423号