当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid Screening of COVID-19 Directly from Clinical Nasopharyngeal Swabs Using the MasSpec Pen
Analytical Chemistry ( IF 6.7 ) Pub Date : 2021-08-25 , DOI: 10.1021/acs.analchem.1c01937 Kyana Y Garza 1 , Alex Ap Rosini Silva 2 , Jonas R Rosa 2 , Michael F Keating 1 , Sydney C Povilaitis 1 , Meredith Spradlin 1 , Pedro H Godoy Sanches 2 , Alexandre Varão Moura 2 , Junier Marrero Gutierrez 2 , John Q Lin 1 , Jialing Zhang 1 , Rachel J DeHoog 1 , Alena Bensussan 1 , Sunil Badal 1 , Danilo Cardoso de Oliveira 2 , Pedro Henrique Dias Garcia 2 , Lisamara Dias de Oliveira Negrini 3 , Marcia Ap Antonio 4 , Thiago C Canevari 5 , Marcos N Eberlin 5 , Robert Tibshirani 6 , Livia S Eberlin 1 , Andreia M Porcari 2
Analytical Chemistry ( IF 6.7 ) Pub Date : 2021-08-25 , DOI: 10.1021/acs.analchem.1c01937 Kyana Y Garza 1 , Alex Ap Rosini Silva 2 , Jonas R Rosa 2 , Michael F Keating 1 , Sydney C Povilaitis 1 , Meredith Spradlin 1 , Pedro H Godoy Sanches 2 , Alexandre Varão Moura 2 , Junier Marrero Gutierrez 2 , John Q Lin 1 , Jialing Zhang 1 , Rachel J DeHoog 1 , Alena Bensussan 1 , Sunil Badal 1 , Danilo Cardoso de Oliveira 2 , Pedro Henrique Dias Garcia 2 , Lisamara Dias de Oliveira Negrini 3 , Marcia Ap Antonio 4 , Thiago C Canevari 5 , Marcos N Eberlin 5 , Robert Tibshirani 6 , Livia S Eberlin 1 , Andreia M Porcari 2
Affiliation
|
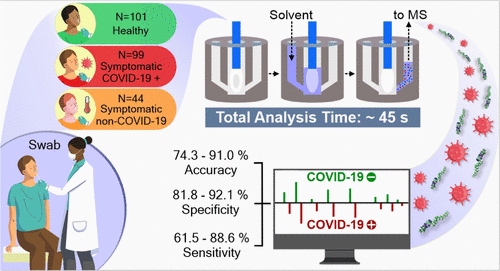
The outbreak of COVID-19 has created an unprecedent global crisis. While the polymerase chain reaction (PCR) is the gold standard method for detecting active SARS-CoV-2 infection, alternative high-throughput diagnostic tests are of a significant value to meet universal testing demands. Here, we describe a new design of the MasSpec Pen technology integrated to electrospray ionization (ESI) for direct analysis of clinical swabs and investigate its use for COVID-19 screening. The redesigned MasSpec Pen system incorporates a disposable sampling device refined for uniform and efficient analysis of swab tips via liquid extraction directly coupled to an ESI source. Using this system, we analyzed nasopharyngeal swabs from 244 individuals including symptomatic COVID-19 positive, symptomatic negative, and asymptomatic negative individuals, enabling rapid detection of rich lipid profiles. Two statistical classifiers were generated based on the lipid information acquired. Classifier 1 was built to distinguish symptomatic PCR-positive from asymptomatic PCR-negative individuals, yielding a cross-validation accuracy of 83.5%, sensitivity of 76.6%, and specificity of 86.6%, and validation set accuracy of 89.6%, sensitivity of 100%, and specificity of 85.3%. Classifier 2 was built to distinguish symptomatic PCR-positive patients from negative individuals including symptomatic PCR-negative patients with moderate to severe symptoms and asymptomatic individuals, yielding a cross-validation accuracy of 78.4%, specificity of 77.21%, and sensitivity of 81.8%. Collectively, this study suggests that the lipid profiles detected directly from nasopharyngeal swabs using MasSpec Pen-ESI mass spectrometry (MS) allow fast (under a minute) screening of the COVID-19 disease using minimal operating steps and no specialized reagents, thus representing a promising alternative high-throughput method for screening of COVID-19.
中文翻译:

使用 MasSpec Pen 直接从临床鼻咽拭子中快速筛查 COVID-19
COVID-19 的爆发造成了前所未有的全球危机。虽然聚合酶链反应 (PCR) 是检测活性 SARS-CoV-2 感染的金标准方法,但替代性高通量诊断测试对于满足普遍测试需求具有重要价值。在这里,我们描述了集成到电喷雾电离 (ESI) 的 MasSpec Pen 技术的新设计,用于直接分析临床拭子,并研究其在 COVID-19 筛查中的用途。重新设计的 MasSpec Pen 系统采用了一次性采样装置,经过改进,可通过直接与 ESI 源耦合的液体提取对拭子尖端进行均匀、高效的分析。使用该系统,我们分析了 244 名个体的鼻咽拭子,包括有症状的 COVID-19 阳性、有症状的阴性和无症状的阴性个体,从而能够快速检测丰富的血脂谱。根据获得的脂质信息生成两个统计分类器。分类器 1 旨在区分有症状的 PCR 阳性和无症状的 PCR 阴性个体,交叉验证准确度为 83.5%,敏感性为 76.6%,特异性为 86.6%,验证集准确性为 89.6%,敏感性为 100% ,特异性为 85.3%。分类器 2 的建立是为了区分有症状的 PCR 阳性患者和阴性个体,包括有中度至重度症状的有症状 PCR 阴性患者和无症状个体,交叉验证准确度为 78.4%,特异性为 77.21%,敏感性为 81.8%。总的来说,这项研究表明,使用 MasSpec Pen-ESI 质谱 (MS) 直接从鼻咽拭子中检测到的脂质谱可以使用最少的操作步骤快速(一分钟内)筛查 COVID-19 疾病,无需专门的试剂,因此代表了有前景的用于筛选 COVID-19 的替代高通量方法。
更新日期:2021-09-21
中文翻译:

使用 MasSpec Pen 直接从临床鼻咽拭子中快速筛查 COVID-19
COVID-19 的爆发造成了前所未有的全球危机。虽然聚合酶链反应 (PCR) 是检测活性 SARS-CoV-2 感染的金标准方法,但替代性高通量诊断测试对于满足普遍测试需求具有重要价值。在这里,我们描述了集成到电喷雾电离 (ESI) 的 MasSpec Pen 技术的新设计,用于直接分析临床拭子,并研究其在 COVID-19 筛查中的用途。重新设计的 MasSpec Pen 系统采用了一次性采样装置,经过改进,可通过直接与 ESI 源耦合的液体提取对拭子尖端进行均匀、高效的分析。使用该系统,我们分析了 244 名个体的鼻咽拭子,包括有症状的 COVID-19 阳性、有症状的阴性和无症状的阴性个体,从而能够快速检测丰富的血脂谱。根据获得的脂质信息生成两个统计分类器。分类器 1 旨在区分有症状的 PCR 阳性和无症状的 PCR 阴性个体,交叉验证准确度为 83.5%,敏感性为 76.6%,特异性为 86.6%,验证集准确性为 89.6%,敏感性为 100% ,特异性为 85.3%。分类器 2 的建立是为了区分有症状的 PCR 阳性患者和阴性个体,包括有中度至重度症状的有症状 PCR 阴性患者和无症状个体,交叉验证准确度为 78.4%,特异性为 77.21%,敏感性为 81.8%。总的来说,这项研究表明,使用 MasSpec Pen-ESI 质谱 (MS) 直接从鼻咽拭子中检测到的脂质谱可以使用最少的操作步骤快速(一分钟内)筛查 COVID-19 疾病,无需专门的试剂,因此代表了有前景的用于筛选 COVID-19 的替代高通量方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号