Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Simple Route toward Next-Generation Thiobase-Based Photosensitizers for Cancer Theranostics
ACS Sensors ( IF 8.2 ) Pub Date : 2021-08-25 , DOI: 10.1021/acssensors.1c01391 Van-Nghia Nguyen 1 , Seonye Heo 1 , Chang Woo Koh 2 , Jeongsun Ha 1 , Gyoungmi Kim 1 , Sungnam Park 2 , Juyoung Yoon 1
ACS Sensors ( IF 8.2 ) Pub Date : 2021-08-25 , DOI: 10.1021/acssensors.1c01391 Van-Nghia Nguyen 1 , Seonye Heo 1 , Chang Woo Koh 2 , Jeongsun Ha 1 , Gyoungmi Kim 1 , Sungnam Park 2 , Juyoung Yoon 1
Affiliation
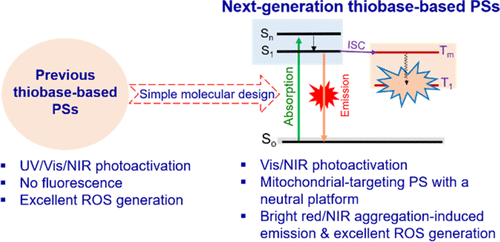
|
Sulfur-substituted biocompatible carbonyl fluorophores have been recognized as effective heavy-atom-free photosensitizers (PSs) for cancer therapy due to their remarkable phototherapeutic properties. However, guidelines on their molecular design are still a substantial challenge. Most of the existing thiocarbonyl-based PSs are nonemissive in both the solution and restricted states, which hinders their further biomedical applications. Herein, we report the interesting finding that sulfur-substituted coumarins exhibit an uncommon phenomenon, aggregation-induced emission. More intriguingly, we also found that the introduction of a strong electron-accepting trifluoromethyl group is crucial to facilitate the mitochondrial-targeting ability of neutral coumarin fluorophores. The resulting CMS-2 PS displayed selective imaging of mitochondria and exhibited much higher photodynamic therapy efficiency toward cancer cells than that of the commercial PS erythrosine B. This work provides deep insight into the molecular design of heavy-atom-free thiobase-based PSs and simultaneously offers a great opportunity to develop novel mitochondrial-targeting fluorescent indicators with neutral bioinspired platforms.
中文翻译:

用于癌症治疗诊断学的下一代基于硫基的光敏剂的简单途径
硫取代的生物相容性羰基荧光团由于其显着的光治疗特性而被公认为有效的无重原子光敏剂 (PSs) 用于癌症治疗。然而,关于其分子设计的指导方针仍然是一个巨大的挑战。大多数现有的基于硫羰基的 PSs 在溶液和受限状态下都是非发射的,这阻碍了它们进一步的生物医学应用。在这里,我们报告了一个有趣的发现,即硫取代的香豆素表现出一种不常见的现象,即聚集诱导发射。更有趣的是,我们还发现引入强的电子接受三氟甲基对于促进中性香豆素荧光团的线粒体靶向能力至关重要。
更新日期:2021-09-24
中文翻译:

用于癌症治疗诊断学的下一代基于硫基的光敏剂的简单途径
硫取代的生物相容性羰基荧光团由于其显着的光治疗特性而被公认为有效的无重原子光敏剂 (PSs) 用于癌症治疗。然而,关于其分子设计的指导方针仍然是一个巨大的挑战。大多数现有的基于硫羰基的 PSs 在溶液和受限状态下都是非发射的,这阻碍了它们进一步的生物医学应用。在这里,我们报告了一个有趣的发现,即硫取代的香豆素表现出一种不常见的现象,即聚集诱导发射。更有趣的是,我们还发现引入强的电子接受三氟甲基对于促进中性香豆素荧光团的线粒体靶向能力至关重要。









































 京公网安备 11010802027423号
京公网安备 11010802027423号