当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DMF-Assisted Iodination Side Reaction during the Preparation of Disulfide Peptides, Its Substrate/Solvent/pH Dependence, and Implications on Disulfide-Peptide Production
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-08-25 , DOI: 10.1021/acs.oprd.1c00151 Yi Yang 1 , Lena Hansen 1 , Aleksandra Fraczek 1 , Fabrizio Badalassi 1 , Johan Kjellström 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-08-25 , DOI: 10.1021/acs.oprd.1c00151 Yi Yang 1 , Lena Hansen 1 , Aleksandra Fraczek 1 , Fabrizio Badalassi 1 , Johan Kjellström 1
Affiliation
|
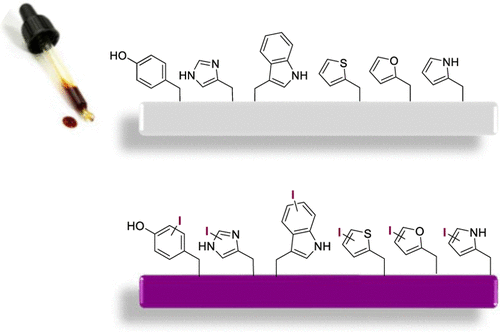
Severe iodination of a disulfide peptide molecule (peptide D) was detected from the on-resin disulfide cyclization process. LC/MS/MS results indicated that iodination occurred on the Thi (thienylalanine) residue. Systematic investigation revealed that the iodination reaction occurred predominantly in the I2/DMF-mediated on-resin disulfide formation reaction. The iodination side reaction was strongly solvent-dependent and favored in DMF and NMP. A tentative mechanism similar to that of the Vilsmeier–Haack reaction was proposed. A variety of amino acid/peptide substrates were tested as iodination substrates. The results indicated the susceptibility of amino acids with electron-rich aromatic groups to iodination. Additionally, various solutions were proposed for preventing peptide iodination based on the findings in this study. This study demonstrates the applicability of the on-resin disulfide production of peptides bearing electron-rich aromatic residues.
中文翻译:

二硫化物肽制备过程中 DMF 辅助的碘化副反应、其底物/溶剂/pH 依赖性以及对二硫化物肽生产的影响
从树脂上的二硫化物环化过程中检测到二硫化物肽分子(肽 D)的严重碘化。LC/MS/MS 结果表明碘化作用发生在 Thi(噻吩基丙氨酸)残基上。系统研究表明,碘化反应主要发生在 I 2/DMF 介导的树脂上二硫化物形成反应。碘化副反应强烈依赖于溶剂,在 DMF 和 NMP 中是有利的。提出了类似于 Vilsmeier-Haack 反应的暂定机制。测试了多种氨基酸/肽底物作为碘化底物。结果表明具有富电子芳族基团的氨基酸对碘化的敏感性。此外,根据本研究的发现,提出了各种解决方案来防止肽碘化。这项研究证明了树脂上二硫化物生产带有富电子芳香族残基的肽的适用性。
更新日期:2021-09-17
中文翻译:

二硫化物肽制备过程中 DMF 辅助的碘化副反应、其底物/溶剂/pH 依赖性以及对二硫化物肽生产的影响
从树脂上的二硫化物环化过程中检测到二硫化物肽分子(肽 D)的严重碘化。LC/MS/MS 结果表明碘化作用发生在 Thi(噻吩基丙氨酸)残基上。系统研究表明,碘化反应主要发生在 I 2/DMF 介导的树脂上二硫化物形成反应。碘化副反应强烈依赖于溶剂,在 DMF 和 NMP 中是有利的。提出了类似于 Vilsmeier-Haack 反应的暂定机制。测试了多种氨基酸/肽底物作为碘化底物。结果表明具有富电子芳族基团的氨基酸对碘化的敏感性。此外,根据本研究的发现,提出了各种解决方案来防止肽碘化。这项研究证明了树脂上二硫化物生产带有富电子芳香族残基的肽的适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号