当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Cyanation of Aryl Thioethers
Organic Letters ( IF 4.9 ) Pub Date : 2021-08-25 , DOI: 10.1021/acs.orglett.1c02285 Tristan Delcaillau 1 , Adrian Woenckhaus-Alvarez 1 , Bill Morandi 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-08-25 , DOI: 10.1021/acs.orglett.1c02285 Tristan Delcaillau 1 , Adrian Woenckhaus-Alvarez 1 , Bill Morandi 1
Affiliation
|
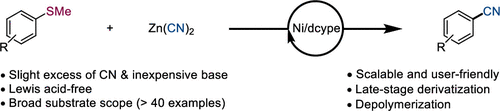
A nickel-catalyzed cyanation of aryl thioethers using Zn(CN)2 as a cyanide source has been developed to access functionalized aryl nitriles. The ligand dcype (1,2-bis(dicyclohexylphosphino)ethane) in combination with the base KOAc (potassium acetate) is essential for achieving this transformation efficiently. This reaction involves both a C–S bond activation and a C–C bond formation. The scalability, low catalyst and reagents loadings, and high functional group tolerance have enabled both late-stage derivatization and polymer recycling, demonstrating the reaction’s utility across organic chemistry.
中文翻译:

芳基硫醚的镍催化氰化
已开发出使用 Zn(CN) 2作为氰化物源的芳基硫醚的镍催化氰化,以获取官能化的芳基腈。配体 dcype(1,2-双(二环己基膦基)乙烷)与碱 KOAc(醋酸钾)的组合对于有效实现这种转化至关重要。该反应涉及 C-S 键活化和 C-C 键形成。可扩展性、低催化剂和试剂负载以及高官能团耐受性使后期衍生化和聚合物回收成为可能,证明了该反应在有机化学中的实用性。
更新日期:2021-09-17
中文翻译:

芳基硫醚的镍催化氰化
已开发出使用 Zn(CN) 2作为氰化物源的芳基硫醚的镍催化氰化,以获取官能化的芳基腈。配体 dcype(1,2-双(二环己基膦基)乙烷)与碱 KOAc(醋酸钾)的组合对于有效实现这种转化至关重要。该反应涉及 C-S 键活化和 C-C 键形成。可扩展性、低催化剂和试剂负载以及高官能团耐受性使后期衍生化和聚合物回收成为可能,证明了该反应在有机化学中的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号