当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coupled Effects of Temperature, Pressure, and pH on Water Oxidation Thermodynamics and Kinetics
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-08-26 , DOI: 10.1021/acscatal.1c02428 Ananth Govind Rajan 1, 2 , John Mark P. Martirez 3 , Emily A. Carter 2, 3, 4
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-08-26 , DOI: 10.1021/acscatal.1c02428 Ananth Govind Rajan 1, 2 , John Mark P. Martirez 3 , Emily A. Carter 2, 3, 4
Affiliation
|
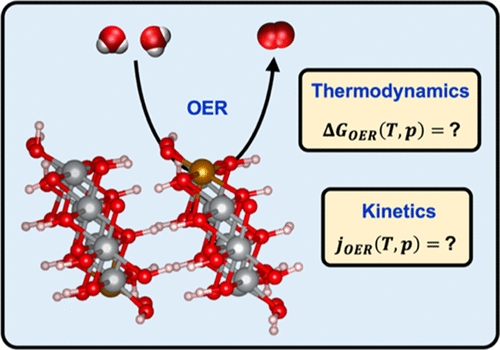
Commercial water-splitting electrolyzers operate at elevated temperature and pressure. Here, we develop a general framework for describing the coupled effects of temperature, pressure, and pH on various phenomena relevant to the oxygen evolution reaction (OER) involved in water splitting. These include water evaporation, water autoionization, and oxygen dissolution. We also consider important variables such as species free energies, species activities, OER standard potentials (EOER0), and rate constants. We apply our model to (Ni,Fe)OOH, a promising electrocatalyst, to study in detail OER thermodynamics and kinetics under realistic operating conditions. We show that an increase in temperature makes water oxidation thermodynamics more favorable with EOER0 decreasing from 1.24 V at 10 °C to 1.18 V at 90 °C. Even this small reduction plays a significant role in accelerating OER kinetics beyond the conventional Arrhenius-type increase in the reaction rate with temperature. Using a recently developed microkinetic model, we show that a room-temperature OER current density of ∼10 mA/cm2 translates to ∼997 mA/cm2 at 90 °C at a fixed potential of 1.51 V versus the reversible hydrogen electrode (RHE). We infer that catalysts on which the room-temperature rate-determining step involves oxygen as a product are favorable for high-temperature operation. Notably, for optimal OER kinetics at elevated temperatures and fixed potentials versus the RHE, the electrolyzer must maintain a pH level less than the standard pH (corresponding to 1 M OH– concentration). Our model predicts an optimal alkali concentration as low as 0.15 mM at 90 °C, with implications for the design of environmentally benign processes. Moreover, we show that a pH of 14.0, often used at room temperature, is physically unachievable at high temperatures. We also demonstrate the mild effect of pressure on the OER potential, with the latter increasing from 1.18 V at 1 bar to 1.21 V at 100 bar, at a fixed temperature of 90 °C. We find the effect of pressure on OER kinetics at fixed potentials to be negligible and indicative of the benefits of maintaining high pressure to produce compressed oxygen (and hydrogen). Our work shows how electrochemical water splitting under operating conditions currently used industrially compares to the process under laboratory conditions.
中文翻译:

温度、压力和 pH 值对水氧化热力学和动力学的耦合影响
商用水分解电解槽在升高的温度和压力下运行。在这里,我们开发了一个通用框架,用于描述温度、压力和 pH 值对与水分解中涉及的析氧反应 (OER) 相关的各种现象的耦合影响。这些包括水蒸发、水自电离和氧气溶解。我们还考虑了重要的变量,例如物种自由能、物种活动、OER 标准电位 ( E OER 0 ) 和速率常数。我们将我们的模型应用于 (Ni,Fe)OOH,一种很有前途的电催化剂,以详细研究实际操作条件下的 OER 热力学和动力学。我们表明,温度的升高使E OER 的水氧化热力学更加有利0从 10 °C 时的 1.24 V 下降到 90 °C 时的 1.18 V。即使是这种小的减少在加速 OER 动力学方面也起着重要作用,超出了反应速率随温度的常规阿伦尼乌斯型增加。使用最近开发microkinetic模型,我们表明〜10毫安/平方厘米的室温OER电流密度2转换为~997毫安/厘米2在 90 °C 和 1.51 V 的固定电位下,与可逆氢电极 (RHE) 相比。我们推断,室温速率决定步骤涉及氧气作为产物的催化剂有利于高温操作。值得注意的是,对于相对于 RHE 在高温和固定电位下的最佳 OER 动力学,电解槽必须保持低于标准 pH 值的 pH 值水平(对应于 1 M OH –专注)。我们的模型预测 90 °C 时的最佳碱浓度低至 0.15 mM,这对环境良性过程的设计具有影响。此外,我们表明通常在室温下使用的 14.0 的 pH 值在高温下是物理上无法实现的。我们还证明了压力对 OER 电位的温和影响,后者在 90 °C 的固定温度下从 1 bar 时的 1.18 V 增加到 100 bar 时的 1.21 V。我们发现压力对固定电位下的 OER 动力学的影响可以忽略不计,这表明保持高压以产生压缩氧气(和氢气)的好处。我们的工作展示了目前工业使用的操作条件下的电化学水分解与实验室条件下的过程相比如何。
更新日期:2021-09-17
中文翻译:

温度、压力和 pH 值对水氧化热力学和动力学的耦合影响
商用水分解电解槽在升高的温度和压力下运行。在这里,我们开发了一个通用框架,用于描述温度、压力和 pH 值对与水分解中涉及的析氧反应 (OER) 相关的各种现象的耦合影响。这些包括水蒸发、水自电离和氧气溶解。我们还考虑了重要的变量,例如物种自由能、物种活动、OER 标准电位 ( E OER 0 ) 和速率常数。我们将我们的模型应用于 (Ni,Fe)OOH,一种很有前途的电催化剂,以详细研究实际操作条件下的 OER 热力学和动力学。我们表明,温度的升高使E OER 的水氧化热力学更加有利0从 10 °C 时的 1.24 V 下降到 90 °C 时的 1.18 V。即使是这种小的减少在加速 OER 动力学方面也起着重要作用,超出了反应速率随温度的常规阿伦尼乌斯型增加。使用最近开发microkinetic模型,我们表明〜10毫安/平方厘米的室温OER电流密度2转换为~997毫安/厘米2在 90 °C 和 1.51 V 的固定电位下,与可逆氢电极 (RHE) 相比。我们推断,室温速率决定步骤涉及氧气作为产物的催化剂有利于高温操作。值得注意的是,对于相对于 RHE 在高温和固定电位下的最佳 OER 动力学,电解槽必须保持低于标准 pH 值的 pH 值水平(对应于 1 M OH –专注)。我们的模型预测 90 °C 时的最佳碱浓度低至 0.15 mM,这对环境良性过程的设计具有影响。此外,我们表明通常在室温下使用的 14.0 的 pH 值在高温下是物理上无法实现的。我们还证明了压力对 OER 电位的温和影响,后者在 90 °C 的固定温度下从 1 bar 时的 1.18 V 增加到 100 bar 时的 1.21 V。我们发现压力对固定电位下的 OER 动力学的影响可以忽略不计,这表明保持高压以产生压缩氧气(和氢气)的好处。我们的工作展示了目前工业使用的操作条件下的电化学水分解与实验室条件下的过程相比如何。



























 京公网安备 11010802027423号
京公网安备 11010802027423号