Urologic Oncology: Seminars and Original Investigations ( IF 2.4 ) Pub Date : 2021-08-26 , DOI: 10.1016/j.urolonc.2021.08.002 Rikiya Taoka 1 , Takashi Kobayashi 2 , Yu Hidaka 3 , Hiroyasu Abe 3 , Katsuhiro Ito 4 , Takahiro Kojima 5 , Minoru Kato 6 , Souhei Kanda 7 , Shingo Hatakeyama 8 , Yoshiyuki Matsui 9 , Yuto Matsushita 10 , Sei Naito 11 , Masanobu Shiga 5 , Makito Miyake 12 , Yusuke Muro 13 , Shotaro Nakanishi 14 , Yoichiro Kato 15 , Tadamasa Shibuya 16 , Tetsutaro Hayashi 17 , Hiroaki Yasumoto 18 , Takashi Yoshida 19 , Motohide Uemura 20 , Manabu Kamiyama 21 , Satoshi Morita 3 , Osamu Ogawa 2 , Hiroyuki Nishiyama 5 , Hiroshi Kitamura 22 , Mikio Sugimoto 1 ,
|
Objective
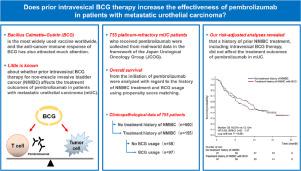
The aim of this study was to determine whether a history of treatment for non-muscle invasive bladder cancer (NMIBC), including intravesical bacillus Calmette-Guerin (BCG) therapy, affects the treatment outcomes of pembrolizumab in patients with metastatic, chemo-resistant urothelial carcinoma (UC).
Materials and methods
The clinicopathological data of 755 patients with metastatic, chemo-resistant UC who received pembrolizumab were retrospectively reviewed. Best overall response and overall survival (OS) from the initiation of pembrolizumab were analyzed with regard to the history of NMIBC treatment and BCG usage using propensity score matching (PSM).
Results
A total of 155 (20.5%) patients had a history of NMIBC treatment, of which 97 (12.8%) had received intravesical BCG therapy. When compared to patients without a NMIBC history (median 10.0 months), the OS from the initiation of pembrolizumab for patients with a NMIBC history (13.3 months, HR [95% CI] 0.79 [0.62–1.02], P = 0.073), those with a NMIBC history and BCG (12.1 months, HR 0.87 [0.64–1.17], P = 0.356), or those with a NMIBC history but not BCG (14.5 months, HR 0.68 [0.45–1.12], P = 0.061) were not significantly different. This tendency was robust after 1:1 or 1:2 PSMs. The objective response rate (ORR, 24.5% vs. 31.0%, P = 0.222) and disease control rate (DCR, 56.1% vs. 52.1%, P = 0.501) of the 155 patients with an NMIBC history did not differ from those of 155 matched patients without an NMIBC history. Among those with an NMIBC history, the prior use of BCG did not affect OS (with vs. without BCG, 12.1 vs. 14.5 months, HR 1.29 [0.80–2.09], P = 0.295), ORR (24.5% vs. 34.0%, P = 0.298) or DCR (57.1% vs. 56.0%, P = 0.908). The ORR in BCG-treated patients was significantly lower than that in those without a NMIBC history (19.8% vs. 33.3%, P = 0.042), whereas DCR between the 2 groups did not differ significantly (55.8% vs. 54.4%, P = 0.855).
Conclusions
Our risk-adjusted analyses revealed that a history of prior NMIBC treatment, including intravesical BCG therapy, did not affect the treatment outcomes of pembrolizumab in metastatic UC patients.
中文翻译:

既往膀胱内卡介苗治疗对帕博利珠单抗治疗转移性尿路上皮癌患者疗效的影响
客观的
本研究的目的是确定非肌层浸润性膀胱癌 (NMIBC) 的治疗史,包括膀胱内卡介苗 (BCG) 治疗,是否会影响 pembrolizumab 在转移性、化疗耐药性尿路上皮癌患者中的治疗结果癌(UC)。
材料和方法
回顾性分析了 755 例接受帕博利珠单抗治疗的转移性、耐药性 UC 患者的临床病理资料。使用倾向评分匹配 (PSM) 就 NMIBC 治疗历史和 BCG 使用情况分析了从 pembrolizumab 开始的最佳总体反应和总体生存期 (OS)。
结果
共有155名(20.5%)患者有NMIBC治疗史,其中97名(12.8%)接受过膀胱内卡介苗治疗。与没有 NMIBC 病史的患者(中位 10.0 个月)相比,有 NMIBC 病史的患者从开始使用派姆单抗开始的 OS(13.3 个月,HR [95% CI] 0.79 [0.62–1.02],P = 0.073),那些有 NMIBC 病史和 BCG(12.1 个月,HR 0.87 [0.64–1.17],P = 0.356),或有 NMIBC 病史但没有 BCG 的患者(14.5 个月,HR 0.68 [0.45–1.12],P = 0.061)没有明显不同。这种趋势在 1:1 或 1:2 PSM 后表现强劲。客观缓解率 (ORR, 24.5% vs. 31.0%, P = 0.222) 和疾病控制率 (DCR, 56.1% vs. 52.1%, P = 0.501) 的 155 名有 NMIBC 病史的患者与 155 名没有 NMIBC 病史的匹配患者没有差异。在有 NMIBC 病史的患者中,先前使用 BCG 不影响 OS(有与无 BCG,12.1 与 14.5 个月,HR 1.29 [0.80-2.09],P = 0.295),ORR(24.5% 与 34.0% , P = 0.298) 或 DCR (57.1% 对 56.0%, P = 0.908)。BCG 治疗患者的 ORR 显着低于没有 NMIBC 病史的患者(19.8% vs. 33.3%,P = 0.042),而两组之间的 DCR 没有显着差异(55.8% vs. 54.4%,P = 0.855)。
结论
我们的风险调整分析显示,既往 NMIBC 治疗史,包括膀胱内 BCG 治疗,不影响转移性 UC 患者中派姆单抗的治疗结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号