当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Use of Interplay between A-Site Non-Stoichiometry and Hydroxide Doping to Deliver Novel Proton-Conducting Perovskite Oxides
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2021-08-26 , DOI: 10.1002/aenm.202101337 Jin Goo Lee 1, 2 , Aaron B. Naden 1 , Cristian D. Savaniu 1 , Paul A. Connor 1 , Julia L. Payne 1 , Jonathan M. Skelton 3 , Alexandra S. Gibbs 4 , Jianing Hui 1 , Stephen C. Parker 5 , John T. S. Irvine 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2021-08-26 , DOI: 10.1002/aenm.202101337 Jin Goo Lee 1, 2 , Aaron B. Naden 1 , Cristian D. Savaniu 1 , Paul A. Connor 1 , Julia L. Payne 1 , Jonathan M. Skelton 3 , Alexandra S. Gibbs 4 , Jianing Hui 1 , Stephen C. Parker 5 , John T. S. Irvine 1
Affiliation
|
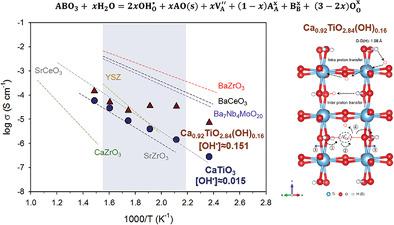
The magnitude of ionic conductivity is known to depend upon both mobility and number of available carriers. For proton conductors, hydration is a key factor in determining the charge–carrier concentration in ABO3 perovskite oxides. Despite the high reported proton mobility of calcium titanate (CaTiO3), this titanate perovskite has thus far been regarded as a poor proton conductor due to the low hydration capability. Here, the enhanced proton conductivity of the defective calcium titanate Ca0.92TiO2.84(OH)0.16 prepared by replacing lattice oxygens with hydroxyl groups via a solvothermal route is shown. Conductivity measurements in a humidified Ar atmosphere reveal that, remarkably, this material exhibits one order of magnitude higher bulk conductivity (10−4 Scm−1 at 200 °C) than hydrated stoichiometric CaTiO3 prepared by traditional solid-state synthesis due to the higher concentration of protonic defects and variation in the crystal structure. The replacement of Ca2+ by Ni2+ in the Ca1−xTi1O3−2x(OH)2x, which mostly exsolve metallic Ni nanoparticles along orthorhombic (100) planes upon reduction, is also demonstrated. These results suggest a new strategy by tailoring the defect chemistry via hydration or cation doping followed by exsolution for targeted energy applications.
中文翻译:

利用 A 位非化学计量和氢氧化物掺杂之间的相互作用来提供新型质子传导钙钛矿氧化物
已知离子电导率的大小取决于迁移率和可用载流子的数量。对于质子导体,水合作用是决定 ABO 3钙钛矿氧化物中电荷载流子浓度的关键因素。尽管报道的钛酸钙 (CaTiO 3 ) 的质子迁移率很高,但由于水合能力低,这种钛酸钙钛矿迄今为止被认为是不良的质子导体。此处,缺陷钛酸钙 Ca 0.92 TiO 2.84 (OH) 0.16增强的质子传导性显示了通过溶剂热途径用羟基取代晶格氧制备的。在加湿 Ar 气氛中的电导率测量表明,该材料显示出比通过传统固态合成制备的水合化学计量 CaTiO 3高一个数量级的体电导率(10 -4 Scm -1在 200 °C)质子缺陷的浓度和晶体结构的变化。Ca 1− x Ti 1 O 3−2 x (OH) 2 x 中的Ca 2+被Ni 2+置换还证明了在还原时主要沿正交(100)平面溶解金属镍纳米颗粒。这些结果提出了一种新的策略,即通过水合或阳离子掺杂来定制缺陷化学,然后针对目标能源应用进行脱溶。
更新日期:2021-10-06
中文翻译:

利用 A 位非化学计量和氢氧化物掺杂之间的相互作用来提供新型质子传导钙钛矿氧化物
已知离子电导率的大小取决于迁移率和可用载流子的数量。对于质子导体,水合作用是决定 ABO 3钙钛矿氧化物中电荷载流子浓度的关键因素。尽管报道的钛酸钙 (CaTiO 3 ) 的质子迁移率很高,但由于水合能力低,这种钛酸钙钛矿迄今为止被认为是不良的质子导体。此处,缺陷钛酸钙 Ca 0.92 TiO 2.84 (OH) 0.16增强的质子传导性显示了通过溶剂热途径用羟基取代晶格氧制备的。在加湿 Ar 气氛中的电导率测量表明,该材料显示出比通过传统固态合成制备的水合化学计量 CaTiO 3高一个数量级的体电导率(10 -4 Scm -1在 200 °C)质子缺陷的浓度和晶体结构的变化。Ca 1− x Ti 1 O 3−2 x (OH) 2 x 中的Ca 2+被Ni 2+置换还证明了在还原时主要沿正交(100)平面溶解金属镍纳米颗粒。这些结果提出了一种新的策略,即通过水合或阳离子掺杂来定制缺陷化学,然后针对目标能源应用进行脱溶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号