当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the formation of carbonates on La2O3 catalysts with OCM activity
Catalysis Science & Technology ( IF 5 ) Pub Date : 2021-08-09 , DOI: 10.1039/d1cy01073e Cairu Guan 1, 2, 3 , Zebang Liu 1, 3 , Danyu Wang 1 , Xiaohong Zhou 1, 3 , Yaoqi Pang 1, 2, 3 , Na Yu 1 , Alexander P. van Bavel 4 , Evgeny Vovk 1 , Yong Yang 1
Catalysis Science & Technology ( IF 5 ) Pub Date : 2021-08-09 , DOI: 10.1039/d1cy01073e Cairu Guan 1, 2, 3 , Zebang Liu 1, 3 , Danyu Wang 1 , Xiaohong Zhou 1, 3 , Yaoqi Pang 1, 2, 3 , Na Yu 1 , Alexander P. van Bavel 4 , Evgeny Vovk 1 , Yong Yang 1
Affiliation
|
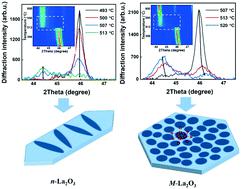
La2O3 catalyzed oxidative coupling of methane (OCM) is one of the promising catalytic partial oxidation processes that converts methane directly into more valuable C2 products. Previous optimization studies found a nanorod shape La2O3 sample (n-La2O3) to exhibit the best low temperature OCM activity. Our previous results correlated the formation of bulk La2O2CO3 with a poisoning effect in OCM. In this study, coupled online MS and in situ XRD are applied to further elucidate this poisoning effect. In the same temperature range, the n-La2O3 sample is compared with a commercial isotropic La2O3 (M-La2O3) catalyst for their OCM performance and propensity to form La2O2CO3 under various CO2 concentrations. The n-La2O3 sample is found to be far more resistant against forming La2O2CO3 than the M-La2O3 sample. In situ XRD results show that after identical exposures to 10%, 30%, and 50% CO2 at around 550 °C, the phase transition to La2O2CO3 is complete for M-La2O3, while n-La2O3 is only partially converted. In addition, coupled online MS and in situ XRD results indicate that the n-La2O3 sample is able to maintain larger grain sizes of La2O3 than the M-La2O3 sample after the same adsorption amount of CO2. Arrhenius plots confirm that in the same temperature range the apparent activation energy for OCM is around 60 kJ mol−1 lower for n-La2O3 than for M-La2O3. These results strongly support that carbonate formation suppresses the OCM performance, which may serve as an indicator in developing more efficient La2O3 based catalysts.
中文翻译:

探索在具有 OCM 活性的 La2O3 催化剂上形成碳酸盐
La 2 O 3催化的甲烷氧化偶联(OCM)是将甲烷直接转化为更有价值的C 2产物的有前途的催化部分氧化过程之一。先前的优化研究发现纳米棒形状的 La 2 O 3样品(n-La 2 O 3)表现出最佳的低温 OCM 活性。我们之前的结果将大量 La 2 O 2 CO 3的形成与 OCM 中的中毒效应相关联。在这项研究中,耦合在线 MS 和原位XRD 被应用于进一步阐明这种中毒效应。在相同温度范围内,n-La 2将 O 3样品与商业各向同性 La 2 O 3 (M-La 2 O 3 ) 催化剂的 OCM 性能和在各种 CO 2浓度下形成 La 2 O 2 CO 3 的倾向进行比较。发现n-La 2 O 3样品比M-La 2 O 3样品更能抵抗形成La 2 O 2 CO 3。原位XRD 结果显示,在同样暴露于 10%、30% 和 50% CO 2 后在大约 550 °C 时,M-La 2 O 3向 La 2 O 2 CO 3的相变完成,而 n-La 2 O 3仅部分转化。此外,耦合在线 MS 和原位XRD 结果表明,在相同的 CO 2吸附量后,n-La 2 O 3样品能够保持比 M-La 2 O 3样品更大的 La 2 O 3晶粒尺寸。. 阿伦尼乌斯图证实,在相同的温度范围内,OCM 的表观活化能约为 60 kJ mol -1n-La 2 O 3比M-La 2 O 3 低。这些结果有力地支持碳酸盐的形成抑制了 OCM 性能,这可以作为开发更有效的 La 2 O 3基催化剂的指标。
更新日期:2021-08-25
中文翻译:

探索在具有 OCM 活性的 La2O3 催化剂上形成碳酸盐
La 2 O 3催化的甲烷氧化偶联(OCM)是将甲烷直接转化为更有价值的C 2产物的有前途的催化部分氧化过程之一。先前的优化研究发现纳米棒形状的 La 2 O 3样品(n-La 2 O 3)表现出最佳的低温 OCM 活性。我们之前的结果将大量 La 2 O 2 CO 3的形成与 OCM 中的中毒效应相关联。在这项研究中,耦合在线 MS 和原位XRD 被应用于进一步阐明这种中毒效应。在相同温度范围内,n-La 2将 O 3样品与商业各向同性 La 2 O 3 (M-La 2 O 3 ) 催化剂的 OCM 性能和在各种 CO 2浓度下形成 La 2 O 2 CO 3 的倾向进行比较。发现n-La 2 O 3样品比M-La 2 O 3样品更能抵抗形成La 2 O 2 CO 3。原位XRD 结果显示,在同样暴露于 10%、30% 和 50% CO 2 后在大约 550 °C 时,M-La 2 O 3向 La 2 O 2 CO 3的相变完成,而 n-La 2 O 3仅部分转化。此外,耦合在线 MS 和原位XRD 结果表明,在相同的 CO 2吸附量后,n-La 2 O 3样品能够保持比 M-La 2 O 3样品更大的 La 2 O 3晶粒尺寸。. 阿伦尼乌斯图证实,在相同的温度范围内,OCM 的表观活化能约为 60 kJ mol -1n-La 2 O 3比M-La 2 O 3 低。这些结果有力地支持碳酸盐的形成抑制了 OCM 性能,这可以作为开发更有效的 La 2 O 3基催化剂的指标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号