当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analytical method development and validation of mucopolysaccharide polysulfate in topical formulations by size exclusion chromatography
Analytical Methods ( IF 2.7 ) Pub Date : 2021-08-25 , DOI: 10.1039/d1ay00673h Gamze Ergin Kızılçay 1 , Sıdıka Ertürk Toker 1 , Dilek Matur 2
Analytical Methods ( IF 2.7 ) Pub Date : 2021-08-25 , DOI: 10.1039/d1ay00673h Gamze Ergin Kızılçay 1 , Sıdıka Ertürk Toker 1 , Dilek Matur 2
Affiliation
|
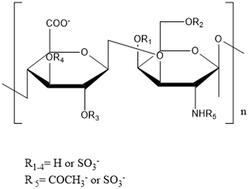
In this study, a new size exclusion chromatographic method has been developed and validated for the analysis of mucopolysaccharide polysulfate used as an anti-inflammatory and antithrombotic agent in topical formulations. Mucopolysaccharide polysulfate was analyzed in Repromer OH-4000 (10 μm, 8.0 × 300 mm) and Repromer OH-5000 (10 μm, 8.0 × 300 mm) columns using a 0.05 M sodium sulfate isocratic elution mobile phase system at 40 °C with a flow rate of 1 mL min−1 and detected by using refractive index detection. The method was validated by means of the limit of quantification, limit of detection, linearity, robustness, recovery, precision and accuracy using the Bioanalytical Method Validation Guidance. The calibration curve showed linearity in the 0.090–1.575 mg mL−1 range. The limits of detection and quantification were found to be 45.000 and 90.000 μg mL−1, respectively. Assay recovery and precision of mucopolysaccharide polysulfate from topical formulations at 0.450, 0.900 and 1.350 mg mL−1 concentrations were evaluated. Intra-day and inter-day relative standard deviation values were calculated to be less than 2.46%. The mean recovery was calculated as 96.64%. The validated method was successfully applied to the determination of mucopolysaccharide polysulfate in cream and gel formulations.
中文翻译:

尺寸排阻色谱法对局部制剂中多硫酸粘多糖的分析方法开发和验证
在这项研究中,开发并验证了一种新的尺寸排阻色谱方法,用于分析在局部制剂中用作抗炎和抗血栓剂的多硫酸粘多糖。在 Repromer OH-4000 (10 µm, 8.0 × 300 mm) 和 Repromer OH-5000 (10 µm, 8.0 × 300 mm) 色谱柱中使用 0.05 M 硫酸钠等度洗脱流动相系统在 40 °C 和流速为1 mL min -1并通过使用折射率检测进行检测。使用生物分析方法验证指南,通过定量限、检测限、线性、稳健性、回收率、精密度和准确度对方法进行了验证。校准曲线在 0.090–1.575 mg mL -1范围内呈线性范围。发现检测限和定量限分别为 45.000 和 90.000 μg mL -1。评估了 0.450、0.900 和 1.350 mg mL -1浓度的局部制剂中多硫酸粘多糖的测定回收率和精密度。日内和日间相对标准偏差值计算为小于 2.46%。平均回收率计算为 96.64%。经验证的方法成功应用于乳膏和凝胶制剂中多硫酸粘多糖的测定。
更新日期:2021-08-25
中文翻译:

尺寸排阻色谱法对局部制剂中多硫酸粘多糖的分析方法开发和验证
在这项研究中,开发并验证了一种新的尺寸排阻色谱方法,用于分析在局部制剂中用作抗炎和抗血栓剂的多硫酸粘多糖。在 Repromer OH-4000 (10 µm, 8.0 × 300 mm) 和 Repromer OH-5000 (10 µm, 8.0 × 300 mm) 色谱柱中使用 0.05 M 硫酸钠等度洗脱流动相系统在 40 °C 和流速为1 mL min -1并通过使用折射率检测进行检测。使用生物分析方法验证指南,通过定量限、检测限、线性、稳健性、回收率、精密度和准确度对方法进行了验证。校准曲线在 0.090–1.575 mg mL -1范围内呈线性范围。发现检测限和定量限分别为 45.000 和 90.000 μg mL -1。评估了 0.450、0.900 和 1.350 mg mL -1浓度的局部制剂中多硫酸粘多糖的测定回收率和精密度。日内和日间相对标准偏差值计算为小于 2.46%。平均回收率计算为 96.64%。经验证的方法成功应用于乳膏和凝胶制剂中多硫酸粘多糖的测定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号