Molecular Therapy ( IF 12.1 ) Pub Date : 2021-08-25 , DOI: 10.1016/j.ymthe.2021.08.028 Xuan Han 1 , Qin Wei 1 , Yan Lv 1 , Ling Weng 1 , Haoying Huang 1 , Qingyun Wei 1 , Mengyuan Li 2 , Yujie Mao 1 , Di Hua 1 , Xueting Cai 1 , Meng Cao 1 , Peng Cao 3
|
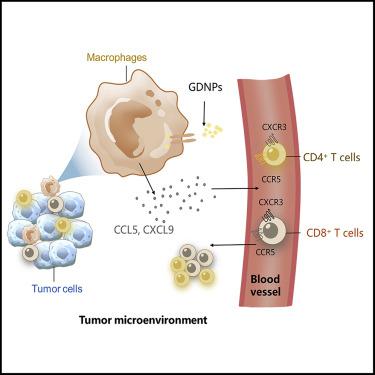
Cold tumor microenvironment (TME) marked with low effector T cell infiltration leads to weak response to immune checkpoint inhibitor (ICI) treatment. Thus, switching cold to hot TME is critical to improve potent ICI therapy. Previously, we reported extracellular vesicle (EV)-like ginseng-derived nanoparticles (GDNPs) that were isolated from Panax ginseng C.A. Mey and can alter M2 polarization to delay the hot tumor B16F10 progression. However, the cold tumor is more common and challenging in the real world. Here, we explored a combinatorial strategy with both GDNPs and PD-1 (programmed cell death protein-1) monoclonal antibody (mAb), which exhibited the ability to alter cold TME and subsequently induce a durable systemic anti-tumor immunity in multiple murine tumor models. GDNPs enhanced PD-1 mAb anti-tumor efficacy in activating tumor-infiltrated T lymphocytes. Our results demonstrated that GDNPs could reprogram tumor-associated macrophages (TAMs) to increase CCL5 and CXCL9 secretion for recruiting CD8+ T cells into the tumor bed, which have the synergism to PD-1 mAb therapy with no detected systemic toxicity. In situ activation of TAMs by GDNPs may broadly serve as a facile platform to modulate the suppressive cold TME and optimize the PD-1 mAb immunotherapy in future clinical application.
中文翻译:

人参衍生的纳米粒子通过重新编程冷肿瘤微环境来增强免疫检查点抗体的功效
以低效应 T 细胞浸润为标志的冷肿瘤微环境 (TME) 导致对免疫检查点抑制剂 (ICI) 治疗的反应较弱。因此,将冷 TME 转换为热 TME 对于改善有效的 ICI 治疗至关重要。以前,我们报道了从人参 CA Mey中分离出的细胞外囊泡 (EV) 样人参衍生纳米颗粒 (GDNP)并且可以改变 M2 极化以延迟热肿瘤 B16F10 进展。然而,冷肿瘤在现实世界中更为常见且更具挑战性。在这里,我们探索了 GDNP 和 PD-1(程序性细胞死亡蛋白-1)单克隆抗体 (mAb) 的组合策略,该策略显示出改变冷 TME 并随后在多种小鼠肿瘤中诱导持久的全身抗肿瘤免疫的能力楷模。GDNPs 增强了 PD-1 mAb 激活肿瘤浸润 T 淋巴细胞的抗肿瘤功效。我们的结果表明,GDNP 可以重编程肿瘤相关巨噬细胞 (TAM) 以增加 CCL5 和 CXCL9 的分泌,从而将 CD8 + T 细胞募集到瘤床中,这与 PD-1 mAb 疗法具有协同作用,且未检测到全身毒性。就地GDNPs 对 TAMs 的激活可以广泛地作为一个简便的平台来调节抑制性冷 TME 并在未来的临床应用中优化 PD-1 mAb 免疫疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号