当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanochemical Synthesis as a Greener Way to Produce Iron-based Oxygen Reduction Catalysts
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2021-08-24 , DOI: 10.1002/zaac.202100194 Marius Gernhard 1 , Max Rautenberg 2 , Gerald Hörner 3 , Birgit Weber 3 , Franziska Emmerling 4 , Christina Roth 5
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2021-08-24 , DOI: 10.1002/zaac.202100194 Marius Gernhard 1 , Max Rautenberg 2 , Gerald Hörner 3 , Birgit Weber 3 , Franziska Emmerling 4 , Christina Roth 5
Affiliation
|
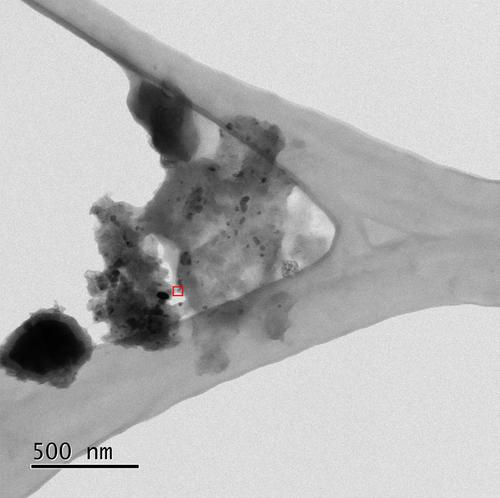
Iron-based catalysts have been reported manifold and studied as platinum group metal (PGM) free alternatives for the catalysis of the oxygen reduction reaction (ORR). However, their sustainable preparation by greener synthesis approaches is usually not discussed. In this work, we propose a new method for the sustainable preparation of such catalysts by using a mechanochemical approach, with no solvents and non-toxic chemicals. The materials obtained from low temperature carbonization (700 °C) exhibit considerable and stable catalytic performance for ORR in alkaline medium. A catalyst obtained from iron hydroxide, tryptophan, dicyandiamide, and ammonium nitrate shows the best electrocatalytic performance with an overpotential of 921 mV vs. RHE at 0.1 mA/cm2 and an electron transfer number of 3.4.
中文翻译:

机械化学合成是生产铁基氧还原催化剂的更环保方法
铁基催化剂已被广泛报道并被研究为用于催化氧还原反应 (ORR) 的无铂族金属 (PGM) 替代品。然而,通常不会讨论通过更绿色的合成方法可持续制备它们。在这项工作中,我们提出了一种使用机械化学方法可持续制备此类催化剂的新方法,无需溶剂和无毒化学品。通过低温碳化(700°C)获得的材料在碱性介质中表现出相当稳定的 ORR 催化性能。从氢氧化铁、色氨酸、双氰胺和硝酸铵获得的催化剂显示出最佳的电催化性能,在 0.1 mA/cm 2时的过电位为 921 mV,而 RHE为 3.4,电子转移数为 3.4。
更新日期:2021-08-24
中文翻译:

机械化学合成是生产铁基氧还原催化剂的更环保方法
铁基催化剂已被广泛报道并被研究为用于催化氧还原反应 (ORR) 的无铂族金属 (PGM) 替代品。然而,通常不会讨论通过更绿色的合成方法可持续制备它们。在这项工作中,我们提出了一种使用机械化学方法可持续制备此类催化剂的新方法,无需溶剂和无毒化学品。通过低温碳化(700°C)获得的材料在碱性介质中表现出相当稳定的 ORR 催化性能。从氢氧化铁、色氨酸、双氰胺和硝酸铵获得的催化剂显示出最佳的电催化性能,在 0.1 mA/cm 2时的过电位为 921 mV,而 RHE为 3.4,电子转移数为 3.4。



























 京公网安备 11010802027423号
京公网安备 11010802027423号